Abstract
Thienopyridines are a class of anti-platelet drugs that are metabolized in the liver to several metabolites of which only one active metabolite can irreversibly antagonize the platelet P2Y12 receptor. Possible effects of these drugs and the role of activated platelets in inflammatory responses have previously been investigated in a variety of animal models, demonstrating that thienopyridines could alter inflammation. Interestingly, P2Y12 may also be expressed in other cells of the immune system, indicating that the effects of the drug could directly target other cells rather than platelets. Furthermore, neutrophil functions could be inhibited by the in vitro generated metabolites of a thienopyridine such as prasugrel, although these cells did not express P2Y12 receptor. Hence, it is not clear whether thienopyridine effects are caused only by the P2Y12antagonism or whether also off-target effects of other metabolites intervene.
To address this question, we investigated P2Y12 deficient mice (KO) during a LPS-induced model of systemic inflammation (3mg/kg for 4 days). In addition, we also treated these KO mice with a thienopyridine drug, clopidogrel (loading dose: 30kg/kg; maintenance dose: 10kg/kg). We investigated possible changes in spleen and bone marrow cell content and lung injury.
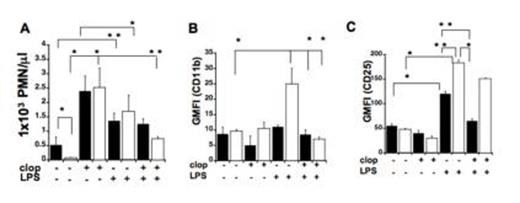
LPS treatments caused accumulation of immune cells in the spleen and altered receptor expression in KO mice. (A) Neutrophil (PMN) content (cell/mL) (B) CD11b and (C) CD25 surface expression (Geometric mean of fluorescence intensity (GMFI)) in spleen samples of WT (black) and KO mice (white) with or without clopidogrel treatments. Values are expressed as Mean ± SEM (**p < 0.05; *p < 0.01; KO LPS-treated versus WT LPS treated and LPS-treated versus untreated, n=6).
LPS treatments caused accumulation of immune cells in the spleen and altered receptor expression in KO mice. (A) Neutrophil (PMN) content (cell/mL) (B) CD11b and (C) CD25 surface expression (Geometric mean of fluorescence intensity (GMFI)) in spleen samples of WT (black) and KO mice (white) with or without clopidogrel treatments. Values are expressed as Mean ± SEM (**p < 0.05; *p < 0.01; KO LPS-treated versus WT LPS treated and LPS-treated versus untreated, n=6).
LPS treatments altered also cell content in the bone marrow of P2Y12KO mice. (A) Neutrophil (PMN) content (cell/mL) (B) CD11b and (C) CD25 surface expression (GMFI) in bone marrow samples of WT (black) and KO mice (white) with or without clopidogrel treatments. Values are expressed as Mean ± SEM (**p < 0.05; *p < 0.01; KO LPS-treated versus WT LPS treated and LPS-treated versus untreated, n=6).
LPS treatments altered also cell content in the bone marrow of P2Y12KO mice. (A) Neutrophil (PMN) content (cell/mL) (B) CD11b and (C) CD25 surface expression (GMFI) in bone marrow samples of WT (black) and KO mice (white) with or without clopidogrel treatments. Values are expressed as Mean ± SEM (**p < 0.05; *p < 0.01; KO LPS-treated versus WT LPS treated and LPS-treated versus untreated, n=6).
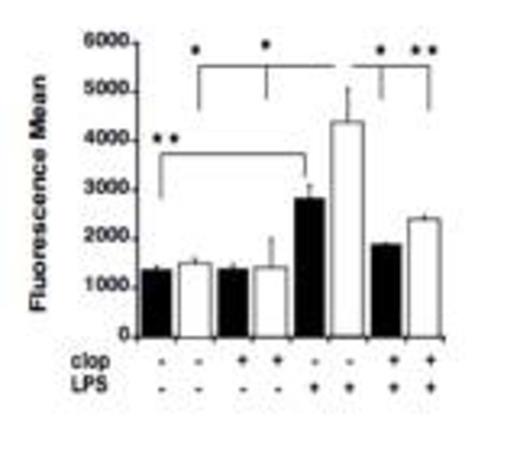
LPS-induced elevations of MPO levels was noted in the lungs of P2Y12null mice. MPO analysis was performed in lung samples of untreated and LPS treated WT (black) and KO mice (white) before and after clopidogrel exposure. Values are expressed as fluorescence mean ± SEM (*p < 0.01; **p < 0.05; KO model versus WT, treated versus untreated; n=3).
LPS-induced elevations of MPO levels was noted in the lungs of P2Y12null mice. MPO analysis was performed in lung samples of untreated and LPS treated WT (black) and KO mice (white) before and after clopidogrel exposure. Values are expressed as fluorescence mean ± SEM (*p < 0.01; **p < 0.05; KO model versus WT, treated versus untreated; n=3).
In conclusion, our study shows that P2Y12 receptor deficiency could enhance LPS-induced inflammation. This finding is in contrast with some previous data presenting clopidogrel treatments as playing a protective role. The observed disparity suggests that the lack of P2Y12 receptor may have different implications in different stages of inflammation. Furthermore, our findings underline that P2Y12 receptor could be involved in regulation of cellular composition even in healthy animals, thus modifying their responsiveness to inflammation. Finally, since clopidogrel treatment could alter KO mice, this drug could have P2Y12 independent effects that need to be further investigated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.