Abstract
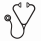
In MDS-004, a phase 3, randomized, double-blind, placebo-controlled multicenter study, the efficacy and safety of LEN was evaluated in RBC transfusion-dependent pts with IPSS-defined Low-/Int-1-risk MDS and del(5q), with or without additional cytogenetic abnormalities (Fenaux P, et al. Blood. 2011;118:3765-76). LEN was recently approved in the European Union (EU) for RBC transfusion-dependent pts with IPSS-defined Low-/Int-1-risk MDS and isolated del(5q). This ad hoc analysis evaluated treatment responses, progression to acute myeloid leukemia (AML), overall survival (OS), and adverse events (AEs) in a subset of pts with isolated del(5q) from the MDS-004 study.
In MDS-004, pts were randomized to either LEN 10 mg/day on days 1–21 or LEN 5 mg/day on days 1–28 of each 28-day cycle; or placebo (PBO). Erythroid response was assessed at 16 weeks. Double-blind treatment continued until unacceptable toxicity, erythroid relapse, or disease progression. PBO or LEN 5 mg pts without response by 16 weeks crossed-over to open-label (OL) LEN 5 mg or 10 mg treatment, respectively. After 52 weeks, pts on double-blind treatment entered the OL phase at their current LEN dose (total study duration 156 weeks). The primary end-point was RBC-transfusion independence (TI) ≥ 182 days. Secondary end-points included duration of RBC-TI ≥ 182 days, cytogenetic response (CyR; IWG 2000), progression to AML, OS, and AEs. In this subset analysis of pts with isolated del(5q) at baseline from the MDS-004 study, RBC-TI ≥ 182 days and CyR were compared across treatment groups (LEN 10 mg vs PBO; and LEN 5 mg vs PBO). Progression to AML and OS were characterized by the Kaplan-Meier method from study randomization with differences evaluated by the log-rank test.
A total of 135 of 205 pts randomized to treatment in the MDS-004 study had isolated del(5q) and were included in the intention-to-treat population for this analysis: LEN 10 mg (n = 47), LEN 5 mg (n = 43), and PBO (n = 45). Baseline characteristics were comparable across treatment groups; median age 69 years (range 36–86), 75% female, and median time since diagnosis 2.5 years (range 0.2–29.2). Median hemoglobin (Hb) level was 8.2 g/dL (range 5.6–11.2) and median transfusion burden was 6 units/8 weeks (range 1–25). Significantly more LEN 10 mg (57.4%) and LEN 5 mg (37.2%) pts achieved RBC-TI ≥ 182 days versus PBO (2.2%; both P < 0.001). Median duration of RBC-TI ≥ 182 days was not reached (NR) for the LEN 10 mg (95% CI 1.6 years–NR) and 5 mg groups (95% CI 0.8 years–NR). Median time to RBC-TI ≥ 182 days response was 4.3 weeks (95% CI 0.3–14.7) and 4.2 weeks (95% CI 0.3–12.3) for the LEN 10 mg and 5 mg groups, respectively. In pts with RBC-TI ≥ 182 days, median maximum Hb increases were 6.5 g/dL (range 8.8–14.4) and 5.4 g/dL (range 8.3–14.1) for the LEN 10 mg and 5 mg groups, respectively. CyR (major + minor response) was achieved in 56.8%, 23.1%, and 0% of pts in the LEN 10 mg, LEN 5 mg, and PBO groups, respectively (LEN 10 mg vs PBO, P < 0.001; LEN 5 mg vs PBO, P = 0.03). Of the pts randomized to PBO, 38 crossed over to LEN. In pts treated with LEN, the estimated 2-year cumulative risk of progression to AML was 13.8%. The rates for the estimated 2-year cumulative risk of progression to AML were 12.6% (95% CI 5.4–27.7), 17.4% (95% CI 8.7–33.3), and 16.7% (95% CI 8.3–32.0) in the LEN 10 mg, LEN 5 mg, and PBO groups, respectively (Figure 1A). Median OS was 4.0 years (95% CI 2.5–NR), 3.5 years (95% CI 1.7–4.8), and 2.9 years (95% CI 2.2–4.2) in the LEN 10 mg, LEN 5 mg, and PBO groups, respectively (Figure 1B). By landmark analysis (6 months), progression to AML was similar (P = 0.9883), but OS was longer (P = 0.0072) in LEN-treated pts who achieved RBC-TI ≥ 182 days versus non-responders. AEs included myelosuppression, with grade 3–4 neutropenia reported in 76.6%, 76.7%, and 17.8% of pts; and thrombocytopenia in 46.8%, 46.5%, and 2.2% of pts in the LEN 10 mg, LEN 5 mg, and PBO groups, respectively.
In this subset analysis of MDS-004 pts with isolated del(5q), LEN therapy was associated with a significant achievement of RBC-TI ≥ 182 days and CyR (57% of pts in the LEN 10 mg group for both RBC-TI and CyR), and had no negative impact on progression to AML or OS. The overall safety profile was well characterized and consistent with the known safety profile of LEN. These data support that LEN is beneficial for the treatment of RBC transfusion-dependent pts with Low-/Int-1-risk MDS and isolated del(5q).
Giagounidis:Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. Mufti:Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Mittelman:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau. Sanz:Celgene Corp.: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau. Platzbecker:Celgene: Honoraria. Selleslag:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding, Speakers Bureau. Beyne-Rauzy:Celgene Corporation: Research Funding; Roche: Research Funding. te Boekhorst:Novartis: Consultancy. del Cañizo:Celgene: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Array: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Guerci-Bresler:Celgene: Honoraria; BMS: Honoraria; Novartis: Consultancy, Honoraria; Amgen: Honoraria. Quesnel:Celgene: Research Funding. Bowen:Celgene: Honoraria. Schlegelberger:Celgene: Consultancy. Fu:Celgene: Employment, Equity Ownership. Benettaib:Celgene: Employment, Equity Ownership. Hellström-Lindberg:Celgene: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Fenaux:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract