Abstract
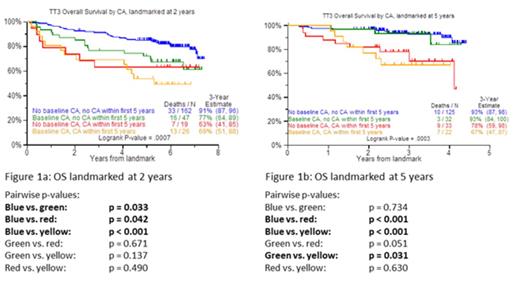
The presence of metaphase cytogenetic abnormalities (CA) has long been considered an adverse prognosticator in multiple myeloma (MM). An update is provided of overall survival (OS) and progression-free survival (PFS) of patients treated with Total Therapy 3 (TT3). The prognostic role of CA was assessed relative to its presence at baseline (BL) and during 2 landmarks (LM) of 2 (LM2) and 5 years of follow-up (LM5). For both LM, the following CA combinations were considered: no CA at BL and up to LM; CA at BL and till LM; CA at BL, no CA till LM; no CA at BL, CA toward LM. Patients considered for evaluation had at least one cytogenetic evaluation prior to the start of therapy and at least one cytogenetic evaluation during follow-up. In Cox modeling, only patients with at least one cytogenetic evaluation prior to baseline were considered. The time-dependent variable indicating whether patients lived to reach a certain LM, and had a CA during the time between enrollment and that LM, was considered along with several typical MM predictor variables. Results showed that, for the LM2, patients with BL-no CA/LM-no CA fared best followed by the BL-CA/LM-no CA combination, with worst outcomes seen in the remainder sharing LM-CA (Figure 1a). In case of the LM5, LM-no CA was superior regardless of BL-CA status, while LM-CA conferred equally poor OS regardless of BL-CA status (Figure 1b). On multivariate analysis, LM5-CA retained the highest HR along with GEP-70-defined high-risk MM, BL-CA and creatinine elevation. We conclude that serial CA monitoring provides a simple cost-effective tool contributing to early detection of disease relapse often without clinical progression (data not shown) that also affords the early detection of MDS-associated CA, an increasing complication of MM therapy. Data will be presented on the detection at BL of GEP features forecasting CA development during 2 and 5 years of follow up.
Disclosures:
Usmani: Celgene: Consultancy, Research Funding, Speakers Bureau; Onyx: Research Funding, Speakers Bureau.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013