Abstract
It is well known that myelodysplastic syndrome (MDS) and acute myeloid leukemia(AML) would develop in patients with multiple myeloma. Many of them are considered as therapy-related MDS/AML (t-MDS/AML). Use of novel agents (NAs) such as bortezomib (Bor), thalidomide (Thal), and lenalidomide (Len) has extended survival of patients with multiple myeloma (MM). However, it is concerned whether a long-term treatment with NAs may increase the risk of the t-MDS/AML. So, we explore whether NAs increase the additional-chromosomal abnormalities in patients with multiple myeloma.
Sequential 350 patients with MM have been treated at JRCMC from 1998 to 2013. 169 patients have been treated with at least one of the NAs (NAs user); namely Bor was administered to 154 patients, Thal 70, and Len 38. The rest of 181 patients had never been treated with either one of the NAs (NAs non-user). Some patients had additional-chromosomal abnormalities (Ad-CAs) associated with t-MDS/AML by -5/5q-, -7/7q-, der(1;7), +8, 13q-, 20q-, inv(3)/t(3;3), t(11q23), der(12p), 11q-, 16q-, t(3;21), der(1;14). We compared the frequency of Ad-CAs detected by total 1112 bone marrow G-banding findings between NAs users and non-users.
Patients' background is not significantly different between NAs users and non-users in terms of age, sex, M-protein type, and ISS stage. 5 patients showed t-AML and 30 patients revealed t-MDS. Ad-CAs was observed in 13 out of 169 NAs users (7.7%): (Bor 11 cases, Thal 10, and Len 5), while 38 out of 181 NAs non-users (21.0%). A cumulative incidence of appearance of Ad-CAs was significantly higher in non-users (P=0.02). In addition, dose and treatment period of Thal and Len was not different between Ad-CAs-positive and negative patients, but not Melphalan (Mel). -7/7q- was the most frequent Ad-CAs (26.3%) of the 38 cases of Ad-CAs in NAs non-users. In contrast, 13q-, 20q-, and +8 were observed in 10 patients (77%), 6 (46%), and 4 (31%) of the 13 cases of Ad-CAs in NAs users, respectively.
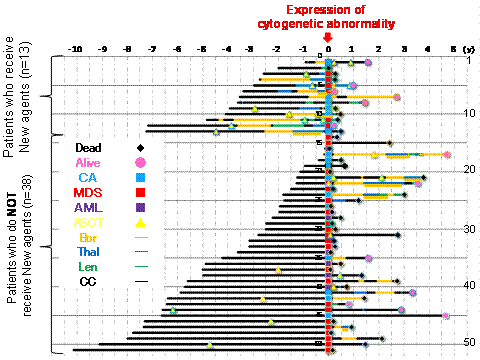
Higher dose administration of Mel was observed amongst patients who represented Ad-CAs. Treatment progress is represented in the Figure. Upper 13 patients were administered NAs, lower 38 patients were not. The time of Ad-CAs is Point 0 year, before and after treatment years are summarized in the Figure. All the patients who developed t-AML died within 2 years of development. t-MDS/AML which develops in patients with multiple myeloma has a poor prognosis and resistance to treatment. We must select chemotherapy regimen taking into account of the risk of developing t-MDS/AML.
The results of the present study demonstrated no evidence of increased risk of Ad-CAs using NAs including Len was observed. It is suggested that Ad-CAs were associated with therapy strategies that were the previous standard before NAs emerged. However, the possibility of an association between +8,13q-,20q- and the use of NAs cannot be excluded. There were drug lag of the approval of NAs issue in Japan; Bor in 2006, Thal in 2008, Len in 2010. Therefore, longer follow-up periods are necessary for an accurate assessment of the risk.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.