Abstract
Achieving allogeneic T-cell depleted BM (TDBM) engraftment under non-myeloablative conditioning represents a potentially safe and attractive approach for generating tolerance as a platform for organ transplantation and for the treatment of non-malignant diseases. However, overcoming the abundant host anti-donor T-cells (HADTC) surviving such protocols remains a major challenge.
The potential role of megadose T cell depleted BM transplants in overcoming this barrier was shown when using 6.0Gy sublethal TBI, but chimerism could not be attained upon further reduction of the conditioning. An alternative approach currently in clinical use is based on the administration of high dose cyclophosphamide at days 3-4 after transplantation. However, attempts have been limited to T cell replete transplants containing at least 2x106 T cells/Kg. The latter are clearly critical for achieving engraftment of mis-matched transplants but they are also associated with a significant risk for GVHD.
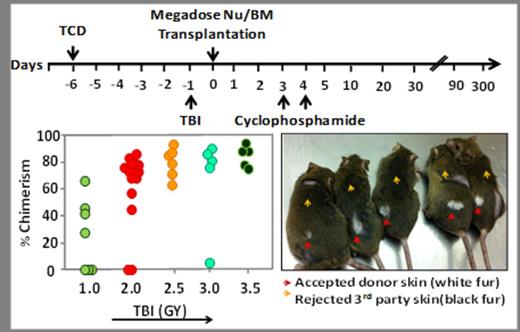
In the present study, we evaluated the potential benefit of combining these two approaches. Initially, regular (5x106) or high dose (25x106) Balb/c-Nude BM cells (fully depleted of T cells by virtue of the 'nude' phenotype) were transplanted (day 0) into fully allogeneic recipients (C3H/Hen). Conditioning included T cell debulking (TCD) with anti-CD4 and anti-CD8 antibodies (300µg each) delivered on day -6, and exposure to 2.0 Gy total body irradiation (TBI) on day -1. High dose Cyclophosphamide (CY) (100mg/kg) was administered on days +3 and +4. Chimerism analysis on days 35, 95, 180 and 225 revealed that none of the BM recipients that were transplanted with a regular dose of 5x106 T depleted BM in the presence or absence of CY, expressed donor type chimerism. In contrast , 4/7 mice receiving 25x106 cells and CY, exhibited (on day 225) more than 47.7±9. % multi-lineage chimerism. The stability of chimerism over more than 7 months indicated that tolerance was likely achieved. Indeed, this was confirmed by subsequent acceptance of donor type Balb/c skin grafts. Thus, 3/4 chimeric mice accepted Balb/c skin and rejected 3rd party C57BL/6 skin, while all mice that were inoculated with regular dose of BM cells in conjunction with CY (7/7), rejected both donor and 3rd party grafts.
Considering previous suggestions by Ildstad et al. that a subset of CD8+ TCR- BM cells is critical for achieving donor type chimerism, we next determined whether such cells are indeed critical for engraftment of megadose T cell depleted BM transplants. To that end, CD8+ cells were depleted from the Balb/c -'nude' mega dose BM preparation, and chimerism induction was compared to control non-depleted 'nude' BM cells. The results of two independent experiments revealed no significant difference (P≥0.9974) in chimerism levels between mice transplanted in the presence (47.3±31, n=16) or absence (47.3±29, n=15) of CD8+TCR- cells, suggesting that such cells are not essential when using megadose T cell depleted transplants in conjunction with CY.
Taken together, our results demonstrate that combination of T cell depleted megadose hematopoietic stem cells with CY administration post transplantation can lead to marked chimerism following low intensity conditioning, in contrast to using each approach alone. Importantly, there is no need to preserve CD8+TCR- cells in the transplant preparation. Such transplants, which are completely free of GVHD risk, could potentially pave the way for a safer tolerance induction modality in organ transplantation and in other non-malignant indications in which the risk associated with GVHD is not justified.
Reisner:Cell Source LTD: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.