Abstract
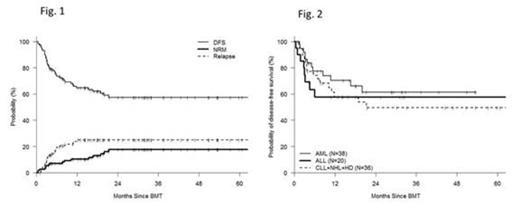
Many patients with hematologic malignancies who may benefit from the curative potential of allogeneic hematopoietic cell transplantation (allo-HCT) will lack a conventional matched sibling (MRD) or matched unrelated (MUD) donor. An HLA-haploidentical related donor is available for nearly all such patients. Attempts to use haploidentical donors that employ stringent ex-vivo T-cell depletion to abrogate GVHD are associated with poor immune reconstitution and high rates of graft rejection. In contrast, the recent use of T-cell replete grafts with post-transplant cyclophosphamide (haplo-ptCy) has resulted in effective control of alloreactivity with low rates of post-transplant infection, graft-rejection and non-relapse mortality using both non-ablative and ablative regimens (Luznik at al BBMT 2008, Solomon et al BBMT 2012.). We recently demonstrated similar outcomes for 53 haplo-PtCy to contemporaneous MRD and MUD transplants performed at our enter (Bashey et al JCO 2013). However there are limited data with respect to the outcome of specific hematologic malignancies using haplo-ptCy. For this study all consecutive patients who underwent haplo-ptCy as a first allogeneic transplant at our center between Oct 2005 and May 2013 (n=115) were included [median age 50 (20-74); M 57%, F 43%; diagnosis- AML(38), ALL(20), CLL(13), NHL(13) HL(10), CML+MPS (12), MDS (7) others (2); graft-BM(58%),PBSC(42%); prior autotranplant(18%); CIBMTR disease risk score high(35%), intermediate(32%) low(33%). Sorror Comorbidity Index-median =1(0-5)]. Preparative regimens used included non-myeloablative [fludarabine 30 mg/m2/d d -6 to -2; TBI 200cGy d-1, Cy 14.5 mg/kg/d on d-6,-5 and 50mg/kg/d on d+3,+4, n=73 ] and myeloablative [regimen 1- same as non-ablative except busulfan 110-130 mg/m2 /d i.v. on d-7 to-3 instead of TBI n=21; regimen 2- fludarabine 30 mg/m2/d d -7 to -5, TBI 150cGy bid x 8 doses (total dose =1200cGy) and Cy 50mg/kg/d d+3,+4 n=21]. Patient characteristics and outcome data were collected prospectively and obtained from our comprehensive database. Survival and disease-free survival (DFS) were estimated using the Kaplan-Meier method. Cumulative incidence of non-relapse mortality (NRM) was calculated using relapse as a competing risk. GVHD was assessed and documented prospectively by a dedicated GVHD nurse. Median CD34+ and CD3+cell doses infused were 4.21 x 10e6/kg (0.84-8.13) and 5.81 x 10e7/kg (1.4-59.5). median time to ANC > 500/mm3 and platelets > 20,000/mm3 were 16 and 26 d. Median % donor chimerism on d 30 were 100% and 100% for PB CD3+ and CD33+ cells respectively. With a median follow-up for living patients of 20 months, the 12 m and 24 m cumulative incidences (CI) of NRM were 10% and 18% and of relapse/progression were 25% and 25% respectively (Fig.1). CI of acute GVHD gd II-IV and gd III-IV at 6 m were 34% and 13% respectively, and of extensive and severe chronic GVHD at 12 m were 40% and 9% respectively. Estimated 12m and 24m probabilities of survival were 73% and 56% and of DFS were 65% and 57% respectively. On a Cox multivariate analysis assessing patient, disease and transplant related covariates, high risk CIBMTR disease score (HR 1.88, p=0.043) and acute GVHD gd 3-4 HR=2.54, p=0.0145) were the only factors significantly associated with survival. For the most frequently treated diagnoses - AML, ALL and mature lymphoid malignancies (CLL, NHL, HL) - the respective estimated 24 m probabilities of DFS were 61%, 58% and 50% respectively (Fig.2). These data demonstrate that haplo-ptCy transplants represent a safe and effective alternative for most patients who may benefit from allo-HCT but lack a conventional donor. They should be offered as standard of care in such patients.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013