Abstract
Clinical studies have shown that exogenously administered culture-expanded human bone marrow mesenchymal stromal/stem cells (BM-MSCs) support hematopoietic cell engraftment and reconstitution after hematopoietic stem cell transplantation (SCT). In addition, these cultured BM-MSCs reveal clinically effective to reduce the side effects of GvHD. Therefore, culture-expanded human BM-MSCs are a promising cell therapy to improve clinical outcome of SCT.
We previously reported that culture-expanded human BM-MSCs support the expansion of hematopoietic cells and pharmacological stimulation of BM-MSCs with parathyroid hormone (PTH) further enhances the BM-MSC-mediated expansion of hematopoietic cells. Here, we show that direct interaction with BM-MSCs is required for the hematopoietic expansion mediated by PTH and cadherin-11 (CDH11) is one of responsible molecules.
When CD34+ hematopoietic progenitor cells (HPCs) were co-cultured with BM-MSCs in StemSpan Serum Free Expansion Medium (StemCell Technologies) supplemented with 100 ng/mL stem cell factor (SCF), 100 ng/mL Flt-3 ligand, 50 ng/mL thrombopoietin (TPO) and 20 ng/mL interleukin (IL)-3, the number of HPCs was increased. The increase in HPCs number by the co-culture with BM-MSCs was significantly enhanced when these MSCs were pre-stimulated with PTH. This enhancement effect of PTH on HPC expansion was not observed in HPC culture alone in the absence of BM-MSCs, which excluded the direct effects of PTH on HPCs. In the presence of a Transwell (Corning), co-culture of HPCs with BM-MSCs stimulated with PTH (BM-pMSCs) did not bring about the enhancement of HPC expansion. This indicates the requirement of direct interaction between HPCs and BM-MSCs. The possible involvement of adhesion molecule(s) in the enhanced HPC expansion by PTH was supported by the observation that the expression of hematopoiesis-associated soluble factors including SCF, CXCL12, and Angiopoietin1 was not altered in BM-pMSCs compared to untreated BM-MSCs (BM-uMSCs).
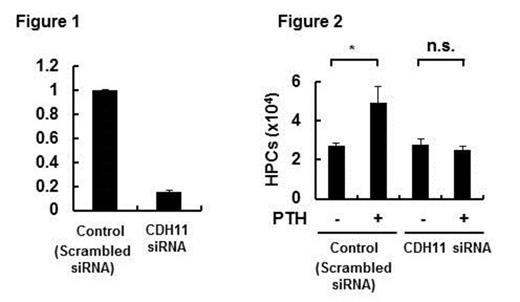
Next, we performed microarray analysis on BM-pMSCs and found CDH11 was upregulated among various adhesion molecules expressed by BM-MSCs. The upregulated expression of CDH11 was confirmed by immunoblotting analysis in which the level of CDH11 in BM-pMSCs was increased by approximately twice. To examine a functional role of CDH11 on BM-MSCs, siRNA experiments were conducted. When the expression of CDH11 mRNA in BM-MSCs was inhibited by CDH11-specific siRNA by around 85% (Figure 1), the enhancement of HPC expansion was inhibited (Figure 2). This inhibition was not observed when BM-MSCs were treated with scramble siRNA.
To further examine a role of CDH11 and PTH on BM-MSCs in hematopoiesis in vivo, bone marrow transplantation experiment was performed. When C57BL/6 mice received suboptimal dose of bone marrow cell transplantation (5 x 104 cells) after lethal irradiation at 10 Gy, the survival rate of mice were about 40%. When PTH was injected subcutaneously to the mice after bone marrow cell transplantation, the survival rate of mice improved to be about 90%. No significant adverse reactions including hypercalcemia were observed after PTH administration. In PTH-treated mice, hematopoietic recover was promoted after lethal irradiation and the following bone marrow transplantation, and the expression of CDH11 in BM-MSC was upregulated compared to the control mice.
In conclusion, CDH11 was a functional and indispensable adhesion molecule associated with the enhancement of HPC expansion by BM-MSCs stimulated with PTH. Pharmacological treatment to target the upregulated expression of CDH11 in BM-MSCs could be a novel strategy to obtain hematopoietic expansion in a clinical setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.