Abstract
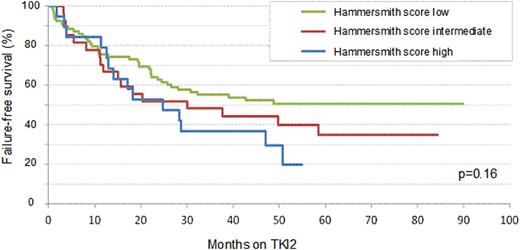
Second generation tyrosine kinase inhibitors (TKI2) have been introduced as 2nd line therapy for chronic myeloid leukemia (CML) failing imatinib (IM) in 2005. In order to optimize the therapeutic strategy and the decisions to be made for chronic phase (CP) CML patients (pts) in this situation, a prognostic score has been established in the Hammersmith hospital (Milojkovic et al. Haematologica 2009) to segregate patients and predict their response to TKI2. However, this score relies on limited data from 2 centers and might not be representative of CP CML pts failing IM. The primary objective of this national study was to retrospectively and prospectively analyse the predictive value of this score in CP CML resistant or intolerant to IM pts from numbers of academic institutions (university and non-university hospitals). All molecular analyses performed locally were standardised according to the IS system since 2005. One hundred and seventy four CP CML cases have been enrolled in 22 centers and their data collected after informed consent, between 2009 and 2012. There were 89 males (51%) and 85 females with a median age at enrolment of 61 (25-86). Sokal score was low for 39%, intermediate for 31% and high for 30 % (5 pts unknown). A minority of patients had IFN-a prior to IM (27%), and none were allotransplanted previously. The median time between diagnosis and IM initiation was 1.4 (0-321) months, IM dose was modified in 138 pts (80%), 98% of pts experienced CHR on IM, 52% CCyR and 29% MMR. Twenty pts (12%) were neutropenic on IM and overall IM duration was 31.2 (0.5-99) months. At TKI2 initiation (63% dasatinib, 37% nilotinib), 128 pts were in CHR (74%), 53 in CCyR (30.5%), 24 in PCyR(14%), 11 (6%) in minor CyR, 13 (7.5%) in minimal and 41 (24%) with no CyR (32 pts: no cytogenetic data available), 137 (79%) pts were in less than major MR, 45.5% harboured a BCR-ABL mutation. One hundred and ten (63%) pts were IM-resistant (molecular 40%, cytogenetic 24.5%, hematologic 8.5%) and 97 (56%) IM-intolerant (considering that some pts can be resistant and intolerant altogether), because of skin, liver, hematologic and gastro-intestinal toxicities mainly. Hammersmith scores (HS) were low (L<1.5) for 89 (65%), intermediate (I) for 28 (20%) and high (H≥2.5) for 20 (15%) pts, incalculable for 37 pts, excluded from the analysis. No statistical difference was observed between the two TKI2 in all variables analysed. Twenty-three percent obtained CHR on TKI2 (3% did not and 74% were already in CHR at TKI2 initiation), 51.5% of evaluable pts were in CCyR at M3, 69% at M6, and 74% at M12; 23% were in MMR at M3, 28% at M6, 36.5% at M12, 8% in MR4.5 at M3, 18% at M6, 19% at M12. At 12 months, 73 pts remained on the first TKI2 with 71 (97%) in CHR, 11 (15%) in CCyR and 31 (42.5%) in MMR, with 47.5% switching to another TKI2 because of resistance (22%) and/or intolerance (19%) to a first TKI2. After a median follow-up of 53 (6-94) months since TKI2 initiation, 2% progressed to accelerated phase, 6% to blast crisis, and 17 (10.5%) died mostly because of CML. A Cox model multivariate analysis demonstrated that a L HS was significantly associated with a better OS (p=0.0062), but not on FFS (p=0.16). Figure 1 shows the failure free survival* to TKI2 according to the HS. Multivariate analyses demonstrated the favourable impact having obtained a CCyR on IM (HR: 2.5, 95%CI: 1.4-4.6, p=0.0021); the negative impact of age 55-65 years at TKI2 initiation (HR: 0.26, 95%CI: 0.1-0.6, p=0.0032) on CCyR rates; and the positive impact of having obtained previous molecular response (≥MMR) on IM (HR: 2.8, 95%CI: 1.79-4.25, p<0.0001) on MMR rates on TKI2. ROC curves and logistic regression analyses demonstrated the predictive value of the HS on CCyR rates (AUC 0.81; HR: 21.4, 95%CI: 4.3-105, p=0.0002) and on ≥MMR rates (AUC: 0.67; HR: 4.5, 95%CI: 2.-10.3, p=0.0003) on TKI2. In conclusion, this retrospective and prospective multicentric analysis performed on large number of pts demonstrated a significant relationship between the HS value and the level of cytogenetic and molecular responses on TKI2 after IM failure and overall survival in the multivariate analysis.
FFS to TKI2 as second line TKI therapy according to Hammersmith score.
*Failure is defined as: No hematologic or cytogenetic response, CHR, CCyR, PCyR MMR or MR4.5 loss, death, progression to AP/BC, definitive TKI2 cessation for resistance or intolerance, allogeneic stem cell transplantation.
Nicolini:Novartis, Ariad, Teva, BMS and Pfizer: Honoraria from Novartis, Ariad, Teva, BMS and Pfizer. Grants from Novartis. Other. Rousselot:BMS, Ariad, Pfizer: Honoraria from BMS, Ariad, Pfizer. Grants from BMS Other. Giraudier:Novartis, BMS: Honoraria. Etienne:Novartis, BMS, Pfizer and Ariad: Honoraria. Roy:Novartis, BMS: Honoraria. Cony-Makhoul:BMS: Honoraria. Marie:BMS: Employment. Mohamed:BMS: Employment. Rea:Novartis, BMS, Teva and Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.