Abstract
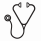
Complete remission (CR) after induction therapy is the first treatment goal in acute myeloid leukemia (AML) patients. The aim of this study is to determine the ability of the Vivia’s novel ex vivo drug sensitivity platform Exvitech analyzing leukemic cell death to predict the CR rates after induction chemotherapy with cytarabine (Ara-C) and idarubicin (Ida) in 1st line AML.
This non-interventional and prospective study included samples from adult patients over 18 years of age diagnosed with de novo AML in Spanish centers from the PETHEMA group. Marrow samples were collected at diagnosis, sent to the Vivia laboratories, and incubated for 48 hours in whole samples in well plates containing Ara-C, Ida, or the combination Ara-C+Ida, each at 8 different concentrations to calculate dose responses. Annexin V-FITC was used to quantify the drug-induced apoptosis. Pharmacological responses are calculated using pharmacokinetic population models. Induction response was assessed according to the Cheson criteria (2003). Patients attaining a CR/CRi were classified as responders. The remaining patients were considered as resistant. Patients dying during induction response assessment were non-evaluable. The correlation was modeled using a generalized additive model with a logit link and a binomial distribution for residuals. Kernel density estimates were then used to plot empirical probability density functions for both groups. Their separation was quantified as the area under the ROC curve and a cut point was selected using the Youden’s criteria to optimize the classification probabilities (sensitivity, specificity). 95% confidence intervals for sampling errors were calculated for all these quantifiers.
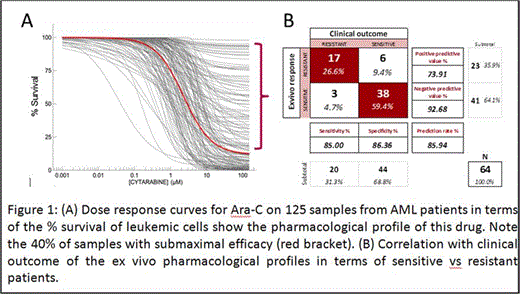
125 patient samples were used to calculate the dose response curves for Ara-C alone, Ida alone, and the synergism of the Ara-C plus Ida combination. For clinical correlation we used 64 patients with a median age of 55 years (range 31 to 72). Dose responses for Ara-C alone are shown in Figure 1.A; note that for many samples there is a significant number (>20%) of resistant cells to Ara-C (bracket). This is a strong clinical predictor of resistance because in the patient the drug will never be present at these high doses for 48 h. The second variable that is a good predictor of response is the synergism between these 2 drugs. The generalized additive model identified an algebraic combination of these 2 variables that yielded the best marker to separate both groups of patients. The probability density functions had minimal overlap. The area under the corresponding ROC curve was 0.965 (0.928, 1.000), and the classification probabilities for the optimal cut point (set at 0.414 for the marker), expressed as percentages, were 85% (62.1% to 96.8%) and 86.4% (72.6% to 94.8%) for sensitivity and specificity, respectively. Results are shown in Figure 1.B; Forty-four patients (68.8%) achieved CR after Ida+Ara-C, and the remaining 20 (31.3%) were resistant. Correlations of the PM test are shown in Figure 1.B. Seventeen of the 20 (85%) patients who fail to achieve CR were predicted as resistance in the ex vivo test. Thirty-eight of the 44 patients (86.4%) who achieved CR showed good ex vivo sensitivity to Ida+Ara-C predicting for CR. When the ex vivo test predicted a patient as sensitive it was correct in 38/39 cases (93%), and when it predicted resistant it was correct 17/23 cases (74%). Overall, 45 patients (86%) had an accurate prediction of their response to treatment.
This study shows that this novel ex vivo pharmacological profile test is able to predict the clinical response to Ida+Ara-C induction. We are increasing the number of patients in this ongoing study, and we are planning a PM Test-adapted Clinical Trial.
Martínez:Vivia Biotech: Employment. Ortega:Vivia Biotech: Employment. Primo:Vivia Biotech: Employment. Hernandez-Campo:Vivia Biotech: Employment. Rojas:Vivia Biotech: Employment. Bennett:Vivia Biotech: Employment. Liebana:Vivia Biotech: Employment. Lopez:Vivia Biotech: Employment. Ballesteros:Vivia Biotech: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract