Abstract
The optimal induction regimen for patients with acute myeloid leukemia (AML) remains unclear. Intensification of daunorubicin (DNR) dose during induction chemotherapy (90 mg/m2) in younger patients with AML has yielded improved complete remission (CR) rates and overall survival (OS) compared to 45 mg/m2. To date, daunorubicin 90 mg/m2 has not been compared to idarubicin for induction in younger patients. Herein, we compare induction outcomes using cytarabine plus either IDA or DNR 90 mg/m2 as induction chemotherapy in patients less than 65 years of age.
This is a single institution case controlled retrospective study evaluating patients aged 18 to 65 years who received induction chemotherapy for AML with either cytarabine 100 mg/m2 x 7 days and DNR 90 mg/m2 x 3 days or cytarabine 200 mg/m2 x 7 days and IDA 12 mg/m2 x 3 days at Moffitt Cancer Center (MCC). Patients who achieved CR were offered consolidation chemotherapy and/or allogeneic transplant as per standard guidelines. Clinical endpoints that were measured and compared include CR, OS and leukemia free survival (LFS). Patients were censored at time of transplant.
Categorical variables were compared using Chi square test, and t- test for continuous variables. Cox regression was used for multivariate analysis. Remission rate were compared using Chi square test. Kaplan-Meier estimates were used for OS and LFS and Log Rank test was used to compare groups. All analyses were conducted using SPSS version 21.
Between January 2005 and April 2013, 175 patients<= 65 years with newly diagnosed AML were included; 84 patients received induction chemotherapy at MCC with high dose DNR and 91 patients received IDA as described above. Baseline characteristics were similar between the two groups (Table 1). The median duration of follow up was 25.9 months.
Baseline Demographics
| . | DNR (n=84) . | IDA (n=91) . | P value . |
|---|---|---|---|
| Median Age - years (range) | 50.5 (18-64) | 57 (18-65) | 0.002 |
| Gender – male | 48 (57%) | 57 (63%) | 0.45 |
| Ethnicity | 68 (81%) | 77 (85%) | 0.61 |
| Caucasian | |||
| Mean Baseline WBC (k/uL) | 24.8 | 20.2 | 0.215 |
| Molecular Markers | 13 (15%) | 17 (19%) | 0.69 |
| FLT3 Positive | 11 (13%) | 19 (21%) | |
| NPM1 Positive | 0.23 | ||
| Risk Status (Cytogenetic/Molecular) | 17 (20%) | 17 (19%) | 0.19 |
| Favorable | 37 (44%) | 38 (42%) | |
| Intermediate | 30 (36%) | 31 (34%) | |
| Unfavorable | 0 (0%) | 5 (6%) | |
| Unknown |
| . | DNR (n=84) . | IDA (n=91) . | P value . |
|---|---|---|---|
| Median Age - years (range) | 50.5 (18-64) | 57 (18-65) | 0.002 |
| Gender – male | 48 (57%) | 57 (63%) | 0.45 |
| Ethnicity | 68 (81%) | 77 (85%) | 0.61 |
| Caucasian | |||
| Mean Baseline WBC (k/uL) | 24.8 | 20.2 | 0.215 |
| Molecular Markers | 13 (15%) | 17 (19%) | 0.69 |
| FLT3 Positive | 11 (13%) | 19 (21%) | |
| NPM1 Positive | 0.23 | ||
| Risk Status (Cytogenetic/Molecular) | 17 (20%) | 17 (19%) | 0.19 |
| Favorable | 37 (44%) | 38 (42%) | |
| Intermediate | 30 (36%) | 31 (34%) | |
| Unfavorable | 0 (0%) | 5 (6%) | |
| Unknown |
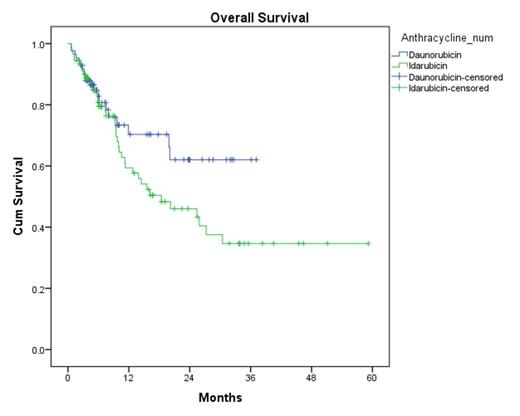
CR rates were similar between the DNR and IDA (74% vs 76%, p = 0.95). Rates of CR after first induction were also similar (61% vs 62%, p = 0.33). The median LFS was 10.2 months with DNR compared to 6.4 months with IDA (p=0.476) (Figure 1). Median OS was not reached in the DNR arm compared to the 18.4 months in the IDA arm (p=0.107) (Figure 2).
Leukemia Free Survival
Overall Survival
No significant differences were observed in the number of patients receiving consolidation chemotherapy (70% vs 66%, p = 0.54) or allogeneic stem cell transplant (23% vs 25%, p = 0.68). There was no difference in 60 day mortality during induction chemotherapy (4.8% vs 5.5%, p = 1.00). In multivariate cox regression analysis anthracycline selection did not affect OS after adjusting for cytogenetic and molecular profile, CR, consolidation received or stem cell transplantation (hazard ratio, 1.25; 95% CI, 0.74-2.09; p = 0.402).
In younger adults with AML, induction chemotherapy with either IDA or DNR yielded similar rates of CR and LFS and OS. However, a trend towards increased overall survival was observed in the DNR cohort, adding further support to the use of high-dose daunorubicin as standard induction for AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.