Abstract
Despite survival benefit provided by current treatments, clinical outcome remains dismal for most patients (pts) diagnosed with multiple myeloma (MM) and novel therapies are greatly needed. Cytokine microenvironment abnormalities, specifically IL-6 constitutive paracrine deregulation, has been implicated in disease pathogenesis and represents a promising therapeutic target in plasma cell dyscrasias. Selective serotonin reuptake inhibitors have been shown to target deregulated cytokines in patients with depression by downregulating TNF and IL-6 levels. In MM treatment, early disease response, defined as at least 50% reduction in m-protein after 2 anti-myeloma therapy (A-MT) cycles, is associated with superior long-term outcomes. In this study, we hypothesized that SSRIs could modulate m-protein normalization when combined with antimyeloma therapies. Herein, we report our single institution retrospective analysis of MM pts treated concurrently with novel therapies and SSRI agents.
After IRB approval, all patients over 18 years diagnosed with MM from January 1, 2000 to December 31st 2012 were identified from the Michael E. Medical Center cancer registry. 39/189 (21%) pts were included for analysis and were considered to be in the selective reuptake inhibitor (SSRI positive) group if by the time of the analysis SSRI (fluoxetine, sertraline, paroxetine, citalopram) were concurrently administered along with A-MT. Pts who did not meet the above criteria were assigned to the SSRI negative group. We analyzed the reduction of m- protein from baseline to completion of 2 cycles of treatment in pts receiving or not receiving concurrent SSRI agents. Association between degree of normalization in both studied groups were analyzed by 2-sample unequal variance t-test with P<0.05 considered as significant. Multivariate Cox regression analysis for intervening variables such as age and initial m-protein fraction size allowed assessment of the impact on magnitude of m-protein reduction in individual treatment groups.
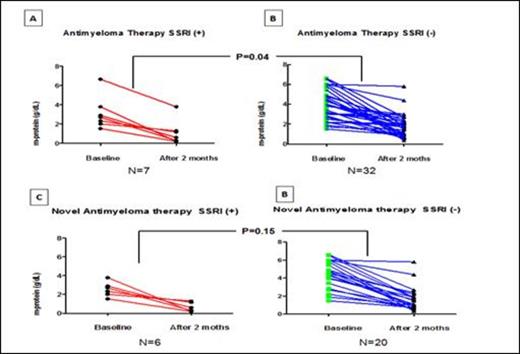
7 and 32 pts were identified as SSRI plus A-MT and only A-MT as of July 31st, 2012, respectively. Initial treatments are depicted in table 1. Median age at diagnosis was 62 years (range, 55-76) and 67 years (range, 39-89) for SSRI+ and SSRI- groups, respectively, P= 0.14, with balanced baseline m-protein level at disease initiation in both groups (Table 1). Median m-protein reduction after 2 cycles of A-MT was 79% and 51% for the SSRI+ and SSRI- group, respectively, P=0.04 (Fig.1 A and B). To adjust for possible confounding effect of less potent not novel A-MT, analysis of m-protein reduction after 2 cycles was performed for pts receiving only novel therapies, SSRI+ (N=6):iMDs plus dexamethasone (dex)[5]; Bortezomib (B) plus dex [1] vs SSRI- (N=20),iMDs plus dex [14]; B+dex [5]; B[1] with median m-protein reduction of 79 % vs. 59% for SSRI+ vs. SSRI-, respectively, P=0.15 (Fig.1 C and D). For pts receiving 2 cycles of SSRI plus A-MT, age ≥ 60 years was favorably associated with deeper normalization of m-protein (P=0.01), which is independent of disease initiation m-protein burden (≥3 g/dL vs ≤ 3g/dL) by multivariate analysis.
Our study showed that more robust m-protein normalization is achieved after 2 cycles of combined A-MT(all therapies) plus SSRIs. A trend toward superior improvement in m-protein normalization was observed when compared to novel A-MT with SSRIs. Our work represents initial preclinical framework that suggests possible role of SSRIs as targeted antimyeloma directed therapy. Larger retrospective studies exploring the role of SSRIs plus A-MT in depth of responses are needed.
Baseline Patient Characteristics
| SSRI+ (N=7) | SSRI- (N=32) | P | |
| Age | |||
| Median, years (range) | 62(55-81) | 67(39-89) | 0.14 |
| Baseline m-protein (g/dL) (range) | 2.7 (2.02-6.65) | 3 (1.5-6.51) | 0.16 |
| Initial Regimen | |||
| Novel Therapy | 5 | 14 | |
| Lenalidomide plus dexamethasone | 1 | 5 | |
| Thalidomide plus dexamethasone | 1 | 9 | |
| Bortezomib plus dexamethasone | 3 | ||
| Bortezomib (single agent) | |||
| Not Novel Therapy | |||
| Melphalan plus prednisone | |||
| Dexamethasone | |||
| SSRI+ (N=7) | SSRI- (N=32) | P | |
| Age | |||
| Median, years (range) | 62(55-81) | 67(39-89) | 0.14 |
| Baseline m-protein (g/dL) (range) | 2.7 (2.02-6.65) | 3 (1.5-6.51) | 0.16 |
| Initial Regimen | |||
| Novel Therapy | 5 | 14 | |
| Lenalidomide plus dexamethasone | 1 | 5 | |
| Thalidomide plus dexamethasone | 1 | 9 | |
| Bortezomib plus dexamethasone | 3 | ||
| Bortezomib (single agent) | |||
| Not Novel Therapy | |||
| Melphalan plus prednisone | |||
| Dexamethasone | |||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.