Abstract
The use of bortezomib has evolved since the APEX study published in 2005. It remains, however, the only study providing a detailed account of the changes in hematological parameters in response to bortezomib-based chemotherapy. The APEX trial enrolled multiple myeloma patients in relapse. In first-line transplant eligible multiple myeloma patients, we use bortezomib twice weekly with low-dose dexamethasone (VelDex) or bortezomib once weekly with dexamethasone and cyclophosphamide (CyBorD). For patients non-eligible to transplant, we use bortezomib once or twice weekly with melphalan and prednisone (VMP). The goal of this study is to describe changes in platelet counts during these three types of bortezomib-based induction therapys.
We conducted a retrospective chart review to examine the complete blood count (CBC) results during treatment with VelDex, CyBorD or VMP. Neutrophil count, hemoglobin and platelet count were measured before every bortezomib infusion. Patients not receiving the anticipated protocol or less than 3 cycles of VelDex or CyBorD or 6 cycles of VMP were excluded from this review.
30 patients were included in this review (VelDex = 11, CyBorD =10, VMP = 9) Results are showed in the following graphs. The use of twice weekly bortezomib is associated with a more pronounced drop in platelets. CyborD once weekly is associated with less severe drop in platelets.
These findings show that once weekly bortezomib is associated with less severe thrombocytopenia. This suggests that the blood monitoring could be less frequent than at each dose of bortezomib. A biweekly monitoring of blood counts could reduce significantly blood tests and waiting time for patients.
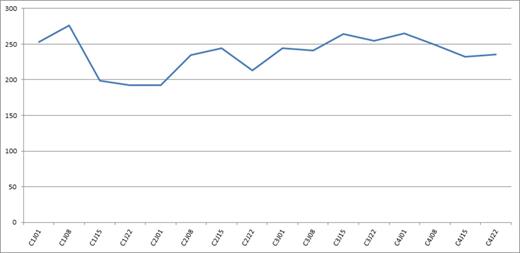
Median platelet count during a once weekly CyBorD treatment
Median platelet count during a once weekly CyBorD treatment
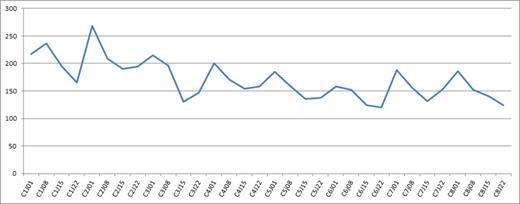
Median platelet count during a twice weekly VelDex treatment
Median platelet count during a twice weekly VelDex treatment
Median platelet count during a once weekly VMP treatment (cycle of 5 weeks)
Median platelet count during a once weekly VMP treatment (cycle of 5 weeks)
Duquette:Janssen: Consultancy, Honoraria. Off Label Use: CyborD is a widely accepted regimen but has not been accepted by the FDA.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal