Abstract
CMV-infection is a serious complication in patients after allogeneic stem cell transplantation (SCT) where immunosuppressive therapy and impaired T cell reconstitution result in a high risk for viral infections. Monitoring of CMV-virus load by PCR and preemptive therapy are important tools to prevent CMV disease. However, CMV specific cytotoxic T cells (CMV-CTLs) are needed to successfully control CMV-infections. CMV-specific multimers composed of the patients HLA Class I molecule bound to CMV pp65 epitopes give the possibility to monitor CMV-CTLs. Here, we present the case of CMV-reactivation following SCT for AML.
The percentage of CMV-specific CD8+ T cells was determined by flow cytometry and mapped to clinical and laboratory parameters of the patient. CD8+ T cells were detected using CD8-fluorescein isothiocyanate (FITC, Beckman Coulter) antibody and CD3 as a T-cell marker was labeled with CD3-allophycocyanin (APC, MACS Miltenyi Biotec) antibody. CMV-specific CD8+ T cells were detected using the CMV major histocompatibility complex (MHC) with Strep-Tactinphycoerythrin (PE) conjugate (Streptamers, IBA GmbH).
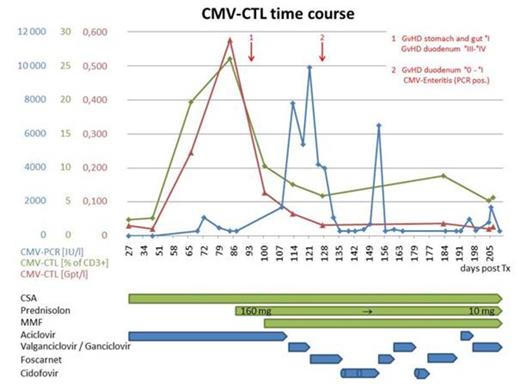
A 60 years old male patient was diagnosed with acute myeloid leukemia (AML) with 95% myeloid blasts in the bone marrow and extramedullary AML manifestations at the time of diagnosis. Following induction therapy the patient was transplanted from a matched unrelated donor. The stem cell recipient as well as his donor had been tested sero-positive for CMV prior to SCT. Within the first month following transplantation, the patient developed an effective CMV specific immunity as seen by high levels of CMV-specific T cells (Figure 1). About three months following transplantation the patient was diagnosed with intestinal GVHD requiring high-dose glucocorticoid treatment. Following steroid exposure, levels of CMV-CTLs dropped and shortly thereafter rising CMV-copy numbers were observed which was accompanied by clinical signs of CMV enteritis. With the administration of antiviral treatment the CMV specific virus load decreased. However, levels of CMV-CTLs remained low, presumably as a result of ongoing steroid exposure.
High levels of CMV-CTLs appeared to control CMV, as seen by a non-detectable virus load in standard PCR testing. The close correlation between the drop in CMV-CTL count and CMV activation highlights the potential of this method to monitor and understand immune responses to CMV following SCT. Of note, early presence of high frequencies of CMV-CTLs did not guarantee CMV-control under steroid exposure as seen in our case. Previous reports have suggested that high dose glucocorticoids may impact CMV-CTLs survival. This is supported by our case, where we see a rapid drop in CMV-CTLs following glucocorticoid exposure. However, the exact molecular mechanisms and more importantly, the predictive value of this finding remain elusive. Furthermore, these data suggest, that patients with ongoing high steroid exposure may not benefit from a transfer of CMV-specific T-cells to control CMV disease.
Further investigations to clarify the potential of CMV-CTL measurements and to understand the effect of steroid exposure at the functional level are warranted. Studies to correlate CMV-CTL counts with the level of immunosuppression and their influence on controlling CMV-disease will follow. In future, this tool could provide a chance to select patients at high risk of CMV reactivation who could profit from an individualized monitoring and early treatment.
Time course of CMV-CTLs after transplantation for AML. High levels of CMV-CTLs appear to control CMV-infection. Following steroid exposure CMV-CTLs decrease and the patient developed a CMV disease with CMV enteritis requiring different antiviral treatment.
Time course of CMV-CTLs after transplantation for AML. High levels of CMV-CTLs appear to control CMV-infection. Following steroid exposure CMV-CTLs decrease and the patient developed a CMV disease with CMV enteritis requiring different antiviral treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.