Abstract
Cord blood (CB) is an increasingly used alternative to bone marrow and mobilized peripheral blood as a source of hematopoietic tissue for transplantation. However, the relatively low cell dose and significantly delayed engraftment when compared to BM and mPB remain significant hurdles. A deficit in the homing of CB hematopoietic progenitor and stem cells (HPSC) to the hematopoietic microenvironment due to suboptimal expression and/or activity of homing molecules is thought to be responsible in part for the delayed engraftment seen with CB in patients. Sialyl Lewis X (sLeX) bearing cells can bind E-selectin, and we have previously reported that ex vivo fucosylation of CB HPSCs with fucosyltransferase VI or VII enhance the rapidity and magnitude of engraftment in mice. Here we explore the engraftment potential of endogenous sLeX bearing CD34+ CB HPSCs to determine if physiologic levels of E-selectin binding predicts engraftment in murine xenografts.
CB cells were sorted with CD34 and HECA-452 (anti-sLex) antibodies. sLeX-bearing CD34+ HPSCs (CD34+HECA+) and CD34+ HPSCs lacking sLeX (CD34+HECA-) were compared phenotypically for stem cell and differentiation markers, by gene expression profiling, E/P/L-selectin binding, colony-forming assays, and for multi-lineage engraftment into NOD-SCID-IL2Rg immune-deficient mice.
Cord blood CD34+HECA+ cells represent 10-20% of CB MNCs and show no significant phenotypic differences from the CD34+HECA- cells in stem cell (CD133, CD90 (Thy-1), CD117 (c-kit), CD143/BB9) and differentiation (CD38, CD33, CD14, CD3, CD19) markers. In agreement, similar percentages of CD34+CD38- and CD34+CD38+ CB cells were found to be HECA+ (18% and 15% respectively, p=0.38), showing no significant bias toward the more immature CD34+CD38- phenotype. mRNA-seq expression analysis revealed relatively few differences in gene expression patterns, although CD34+HECA+ cells express higher levels of the gamma globin genes HBG1 and HBG2, the components of fetal hemoglobin. As predicted, CD34+HECA+ cells demonstrated significantly increased ability to bind recombinant E-selectin in vitro, with no differences in P- and L-selectin binding. Importantly, colony forming assays revealed a small (30%) disadvantage to the CD34+HECA+ cells revealing that the CD34+HECA+ CB cells do not have enriched stem cells activity by CFU assay.
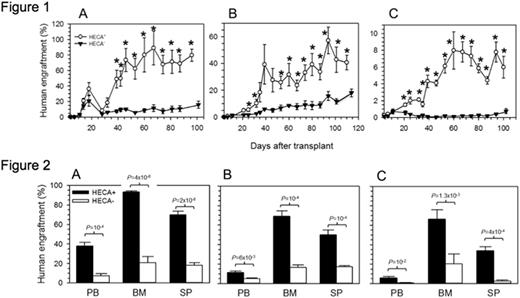
However, CD34+HECA+ cells demonstrated significantly higher rate and magnitude of engraftment when compared to CD34+HECA- cells in three independent NSG experiments (Figure 1). Indeed bone marrow, peripheral blood and splenic levels of human hematopoietic cells were consistently 3-5-fold higher in CD34+HECA+ injected mice than in CD34+HECA- injected controls (Figure 2). Multi-lineage engraftment data reveals higher levels of myeloid (CD33+/CD14+), B-lymphocytes (CD19+/CD20+) and platelets (6-14-fold, CD41a+/CD61+) in CD34+HECA+ cells, but interestingly lower levels of T-lymphocytes (CD3+). Finally, secondary transplants had equal magnitude of engraftment, indicating no bias in short- versus long-term HSPCs.
Data presented here supports the hypothesis that endogenous sLex levels on CD34+ cells is associated with enhanced engraftment rapidity and magnitude but that this is not reflective of an enriched stem cell fraction. Rather it appears to be an indicator of homing to the bone marrow through E-selectin binding. Functional separation of stemness and homing supports the approach to improve CB transplantation through decoration of CB cells with sLex via ex vivo fucosylation (see clinical trial abstract by Popat and Shpall)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

