Abstract
We previously reported strong power to predict relapse and lower event free survival (EFS) associated with the presence of minimal residual disease (MRD) > 0.1% detected in bone marrow (BM) both pre- and post-transplant by standardized multi-channel flowcytometry in children with ALL enrolled on a randomized phase III Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial, ASCT 0431. We performed further analysis of this cohort to test whether the high level of sensitivity of MRD detection offered by deep-sequencing methods increases predictive power for relapse and event free-survival (EFS). Patients in the trial were children aged 1-21yrs who underwent TBI-based myeloablative HCT for high risk ALL in CR1 or CR2. BM samples were taken within 2 weeks of initiation of the preparative regimen (pre-HCT sample) and at 1,3, and 9-12 months after HCT. These samples were sent for central flow cytometry and banked.
DNA was prepared from marrow specimens at each time point in a patient, and used as template in preparing IGH VDJ sequencing reactions. 20,000 B cell genomes were used as input for index specimen libraries, used to define IGH sequences greater than 5% frequency in the sample. For a large majority of patients the index sample was taken at diagnosis, prior to transplant, but in five patients this specimen was unavailable, so a specimen taken at relapse was used to define the tumor tagging sequences. 130 bp reads starting at the WGXG motif in the J segment and extending back across the CDR3 region into the V segment were collected in each library, to a coverage of at least 5X for the input genomes. Tumor tagging sequences were confidently identified in 64 patients. To assess MRD, we then prepared libraries from the equivalent of 100,000 B cell genomes at each time point during or after transplant, and screened the resulting data for the frequency of each tumor tracking sequence in the individual.
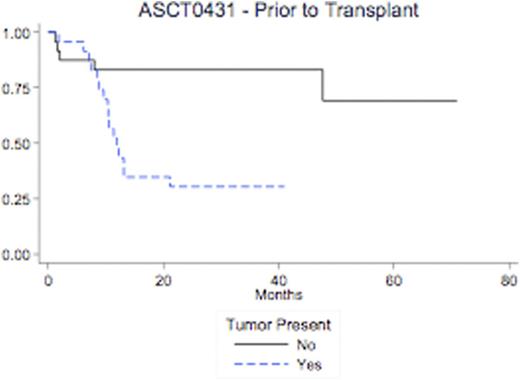
The Kaplan-Meier estimate of EFS and the Gray estimate of cumulative incidence of relapse were calculated. Evidence of tumor in the pre-transplant sample had significantly increased 36-month cumulative incidence (CI) of relapse when compared with no evidence tumor (57% v. 4.4%; p=0.008). Similarly, evidence of tumor was associated with a significant increase in 36-month CI of relapse or death when compared with no evidence of tumor (70% v. 17%, p=0.0014; and 54% v. 17%, p=0.012). Evidence of tumor in the post-transplant sample (within 90 days of transplant) had significantly increased 36-month cumulative incidence (CI) of relapse when compared with no evidence tumor (75% v. 26%; p<0.001). Similarly, evidence of tumor was associated with a significant increase in 36-month CI of relapse or death when compared with no evidence of tumor (83% v. 33%, p=0.0014; and 59% v. 27%, p=0.012).
Patients with no detectable leukemia by deep sequencing pre-HCT relapsed less that 5% of the time, a striking improvement compared to our previous ability to predict relapse with flow MRD (No detectable MRD by flow, 25% risk of relapse in the previous analysis). Detection of any tumor in the first 90 days after HCT by this method is also highly predictive of relapse and survival. Patients identified as high risk for relapse by this method may be candidates for interventions aimed at preventing relapse. Analysis including a direct comparison with flow MRD and post transplant chimerism, as well as definition of specific cut points for levels of disease detected by deep sequencing will be presented with this data.
Carlson:Adaptive Biotechnologies: Consultancy, Equity Ownership, Patents & Royalties. Kalos:Novartis corporation: CART19 technology, CART19 technology Patents & Royalties; Adaptive biotechnologies: Member scientific advisory board , Member scientific advisory board Other. Cindy:Adaptive Biotechnologies: Employment. Williamson:Adaptive Biotechnologies: Employment. Grupp:Novatis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.