Abstract
Sickle Cell Anemia (SCA) remains a disease with high risk of morbidity and early death. HSCT is currently the only curative treatment for SCA, but its use has been limited by the risks of transplant-related mortality (TRM) and GVHD. We have previously demonstrated that antithymocyte globulin (ATG) efficiently decreases the rejection rate in HSCT for SCA; however, its optimal dose has not been defined, prompting us to retrospectively analyse outcomes in patients from the SFGM-TC cohort receiving different ATG doses.
The cohort includes 236 SCA-patients (109F, 127M), (229 SS, 5 Sb0, 2 SDPunjab) transplanted in France (1988-2012) with geno-identical donors after homogeneous myeloablative conditioning regimen (CR; BU-CY), and rabbit ATG (n=215) at 5-15 mg/kg (n=35), 20 mg/kg (n=160) or no ATG (n=20). Cell sources were bone marrow (BM, n=197), cord blood (CB, n=30), CB+BM (n=8), and peripheral blood cells (PBC, n=1). Chimerism was assessed by quantitative real-time PCR from PBC at Day-30, 60, 90, Month-6, 12 and every year post-transplant, and defined as total donor chimerism (TDC): >95% donor (D); low (5-50%D) or high (50-95%D) mixed chimerism (MC),; or rejection (< 5%D). Disease-free survival (DFS) was defined as the absence of HSCT-related death and rejection. GVHD prophylaxis consisted of CSA-short MTX for BMT and CSA alone for CBT.
Results
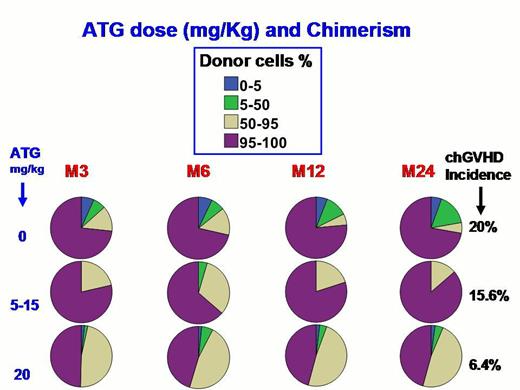
Mean age at transplant was 9.7yr (range: 2.2-28.9), with 33 patients older than 15. With a mean follow-up of 5.6yr (range 0.5-22.5), TRM was not different with or without ATG (3.4%; 0.8-6%) whereas DFS was higher (p=0.004) with ATG (95.4%; 92-4-98.4) than without (72.7%; 51.7-93.7), due to differences in rejection rates (p<0.001). DFS was similar whether the cell source was CB or BM. No lymphoma was observed, and only one post-transplant death at 5m was virus-related (adenoviral meningo-encephalitis). Incidences of AGVH ≥2, 3-4 and chronic-GVHD (cGVHD) were 19%, 6% and 8.7%, respectively. Univariate Cox analysis showed that significant risk factors affecting DFS were low dose of ATG, AGVH grade, and cGVHD, whereas cell source, ABO compatibility, donor’s and recipient’s (DR) age, and CMV status were not. Risk factors associated with cGVHD (Logistic regression) were ATG dose (20% incidence with no ATG, 15.6% with 5-15 mg/kg and only 6.4% with 20), DR age (even after exclusion of CBT), whereas DR genders, DR CMV status, and cell source doses (NC, CD34, CD3+) were not. Multivariate analysis only retained ATG dose (OR=0.9/mg/kg increase, 95% CI:0.8-1, p=0.007) and donor’s age>15 (OR=7.9, 95%CI: 2.5-25.6,p<0.001) as significant independent risk factors for cGVHD. Chimerisms (n=165) (Figure) were significantly associated with ATG dose and cGVHD occurrence. Among the 6 patients with low MC at 1yr, 3 had not received ATG and rejected at 2, 2.2 and 8yr post-HSCT whereas the 3 others who had received ATG remained stable. All patients with high MC at 1yr (n=54) and TDC (n=80) are doing well with the same electrophoretic profile as their donor, excepted one patient who experienced appearance of MC with hemolysis (D/R group U+/U-) 5yr post-CBT, requiring donor lymphocyte infusion (DLI), which reversed chimerism to TDC but induced cGVHD.
This study confirms that myeloablative CR with ATG offers 95% DFS to SCA-patients and that stable MC in absence of SCA-symptoms does not require intervention such as DLI. It reports for the first time in HSCT for SCA that donor’s age significantly increases GVHD risk, suggesting to choose the youngest sibling when possible, and that high ATG dose (20 mg/kg), which significantly reduces cGVHD risk without enhancing viral risk, should be recommended.
Bernaudin:Novartis: Research Funding. Bertrand:ERYTECH Pharma: Principal Investigator Other. Gluckman:Cord use: Honoraria; gamida: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.