To the editor:
Platelets are known to promote coagulation in a factor XII (FXII)-dependent manner.1 Consistent with earlier studies that identified synthetic platelet-size polyphosphates (polyP) as FXII activators in vitro,2 we have shown that platelet polyP of 60 to 100 phosphate subunit lengths activate FXII in vivo, thus contributing to thrombus propagation.3 Independent laboratories have confirmed these findings4,5 and have shown that the ability of polyP to generate FXIIa in vitro increases with the chain length of the polymer.6 Inhibition of polyP interferes with thrombin receptor agonist peptide (TRAP)-activated platelet driven-coagulation.7 In sharp contrast, Faxälv et al postulate in their recent paper that platelet-driven coagulation is unrelated to FXII and question the importance of platelet polyP as FXII activators.8
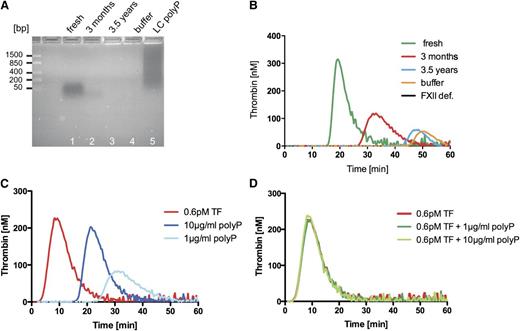
The data described in Faxälv et al rely on a single polyP sample that was purified in December 2009 from human platelets and supplied to the authors by our laboratory. Although we cannot provide concrete evidence as to the timing of their experiments, the authors do not characterize their used polyP anywhere in the manuscript. Here, we compare freshly isolated platelet polyP, platelet polyP stored for more than 3 months, and stocks of polyP stored for 3.5 years, similar to the preparation of polyP originally provided to Faxälv et al. The polyP-sensitive 4,6 diamidino-2-phenylindole (DAPI) dye readily stains freshly isolated platelet polyP and long-chain synthetic polyP. The DAPI signal is largely reduced in the 3-month-old polyP sample and becomes undetectable in the 3.5-year-old-sample, indicating that the polymer time-dependently gets degraded and has significant alterations in its structure (Figure 1A). Independent studies show that platelet polyP degrades over a period of months, even when stored at −80°C.9 We confirmed that intact platelet polyP function as FXII activators by performing real-time thrombin formation on freshly isolated polyP samples and the polyP sample at the time of shipment to Dr. Lindahl’s laboratory. We show that intact material is procoagulant and generates thrombin in an FXII-dependent manner (Figure 1B). Procoagulant activity is reduced in a 3-month-old sample and disappears in a 3.5-year-old sample. Use of degraded polyP offers an explanation for the negative results shown by Faxälv et al, and explains the observation that the authors were unable to dissolve their polyP. Various independent laboratories have successfully dissolved platelet polyP at higher concentrations, and synthetic platelet-size polyP is used in technical processes in extremely high concentrations. Cumulatively, the data shown by Faxälv et al were obtained from experiments using undefined, most likely degraded polyP, which has led to misleading results caused by an artifact.
Time-dependent degradation and tissue factor (TF) addition blunt platelet polyP procoagulant activity. (A) Analysis of polyP stored for various times: 60 ng/lane of polyP was ran on a 2% agarose gel and visualized by negative DAPI staining. Lane 1 indicates freshly isolated platelet polyP; Lane 2, polyP stored for 3 months; Lane 3, 3.5-year-old platelet polyP (as provided to Faxälv et al); Lane 4, buffer; and Lane 5, long chain polyP. A molecular-weight DNA standard is given to the left. (B) Time-dependent loss of polyP procoagulant activity: real-time thrombin formation with fluorogenic substrate in normal human platelet-poor plasma triggered by the addition of 10 µg/mL of freshly prepared platelet polyP, platelet polyP stored for 3 months, 3.5-year-old polyP, and vehicle control (buffer). Thrombin formation triggered by fresh platelet polyP in FXII plasma is blotted for control (FXII def.). (C) Platelet polyP-triggered (1, 10 µg/mL) and TF-triggered (0.6 pM) thrombin formation in platelet-poor plasma. Agents were applied separately. (D) Thrombin generation by TF and a combined application of TF-platelet polyP. Graphs are representative of n = 5 experiments.
Time-dependent degradation and tissue factor (TF) addition blunt platelet polyP procoagulant activity. (A) Analysis of polyP stored for various times: 60 ng/lane of polyP was ran on a 2% agarose gel and visualized by negative DAPI staining. Lane 1 indicates freshly isolated platelet polyP; Lane 2, polyP stored for 3 months; Lane 3, 3.5-year-old platelet polyP (as provided to Faxälv et al); Lane 4, buffer; and Lane 5, long chain polyP. A molecular-weight DNA standard is given to the left. (B) Time-dependent loss of polyP procoagulant activity: real-time thrombin formation with fluorogenic substrate in normal human platelet-poor plasma triggered by the addition of 10 µg/mL of freshly prepared platelet polyP, platelet polyP stored for 3 months, 3.5-year-old polyP, and vehicle control (buffer). Thrombin formation triggered by fresh platelet polyP in FXII plasma is blotted for control (FXII def.). (C) Platelet polyP-triggered (1, 10 µg/mL) and TF-triggered (0.6 pM) thrombin formation in platelet-poor plasma. Agents were applied separately. (D) Thrombin generation by TF and a combined application of TF-platelet polyP. Graphs are representative of n = 5 experiments.
Faxälv et al evaluate polyP-initiated and FXII-mediated coagulation, yet they analyze polyP/FXII-triggered clotting in samples that they supplement with 0.6 pM of TF. Previous studies have shown that presence of 0.6 pM of TF initiates clotting independently of FXII and shortens clotting times by >250%.10 We analyzed the procoagulant effects of platelet polyP in the presence of TF (0.6 pM), as used by the authors. Platelet polyP and TF alone readily initiated thrombin formation in human plasma (Figure 1C). Simultaneous stimulation with TF and platelet polyP did not increase thrombin formation any further than 0.6 pM TF did. Real-time thrombin formation triggered by TF and TF-platelet polyP (1 and 10 µg/mL) is identical (Figure 1D). The experimental setting used by Faxälv et al is not sensitive in assessing platelet polyP/FXII-mediated coagulation; thus, the experiments shown in their Figure 4 and supplemental Figure 2 do not allow for conclusions to be drawn regarding FXII activation by platelet polyP.
Faxälv et al claim that our platelet activation data are “irreproducible.” The authors previously published that TRAP-driven platelet activation shortened whole blood clotting times by >2.5-fold (1440 ± 300 seconds to 520 ± 80 seconds in TRAP-6–stimulated blood11 ). In contrast, Faxälv et al now show that TRAP-6 is ineffective in shortening clot times (600 ± 300 seconds compared with 400 ± 75 seconds in TRAP-6–stimulated blood). In their current study, Faxälv et al show that the potent platelet activator A23187 has scarce impact on clot times in whole blood (7.5 ± 1.0 minutes without A23187-stimulated blood vs 6.2 ± 1.0 minutes in A23187-stimulated blood), in contrast to their previous work using the same experimental setup (a ReoRox instrument).11 Given that the authors are unable to reproduce their own data, it seems inappropriate to claim other studies to be irreproducible.
The authors prepare platelet-free plasma using a Minisart filter made of polytetrafluoroethylene that triggers artificial contact activation.12 This is evident in Figure 3 of their paper: without TF or FXII activator, the addition of phospholipids and Ca2+ generates thrombin, indicating the presence of FXIIa. Indeed, the FXIIa inhibitor corn trypsin inhibitor blocks the filter-produced FXIIa. Furthermore, the authors use Boc-Gln-Gly-Arg-AMC to measure FXIIa. Boc-Gln-Gly-Arg-AMC is cleaved by >20 other plasma proteases with comparable specificity, kinetics, and affinity compared with FXIIa and is readily cleaved in plasma in the absence of FXII.13 Cumulatively, the data presented by Faxälv et al in their Figures 1, 2, and 3 are consistent with an artifact effect of filter-induced FXII activation measured with an inappropriate substrate.
We highly regard scientific discussions that improve the quality of this field. However, the study by Faxälv et al has significant technical and logical limitations, is in conflict with an array of previous studies (including the authors’ previous work), misinterprets data, and cumulatively fails to present experimental support for its bold claims that platelet polyP do not function as FXII activators.
Authorship
Acknowledgments: This work was supported in part by grants from Vetenskapsrådet (K2013-65X-21462-04-5), Hjärt Lungfonden (20110500), Stockholms läns landsting (ALF, 2110471), Cancerfonden (100615), and a European Research Council grant (ERC-StG-2012-311575_F-12) to T.R.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: K.F.N. and H.M.S. performed research; H.M.S., N.J.M., and T.R. analyzed data and designed the experiments; and T.R. wrote the manuscript.
Correspondence: Thomas Renné, Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital Solna (L1:00), SE-171 76 Stockholm, Sweden; e-mail: thomas@renne.net.