To the editor:
Recent genome-wide association studies (GWASs) of hematological traits have consistently found strong associations between the HBS1L-MYB intergenic region on 6q23 and a number of clinically significant hematologic traits, including fetal hemoglobin (HbF) levels, red blood cell counts and size, platelet counts, and white blood cell counts.1-4 Moreover, the variants in this locus have been shown to strongly affect the severity of sickle cell disease and thalassemia, emphasizing their clinical importance.2,5-7 The associated region is intergenic between 2 genes: HBS1L and MYB.1,2 Rare coding variants in MYB have been shown to be associated with HbF levels,2 whereas other evidence has suggested that variants in this region may affect HBS1L expression.8 Therefore, as is the case with many loci implicated from GWASs, the exact biological basis for the alteration of hematologic parameters remains unclear. A greater understanding of the genes underlying the clinical effects of this locus holds tremendous promise for the development of mechanism-based therapies for the hemoglobin disorders.
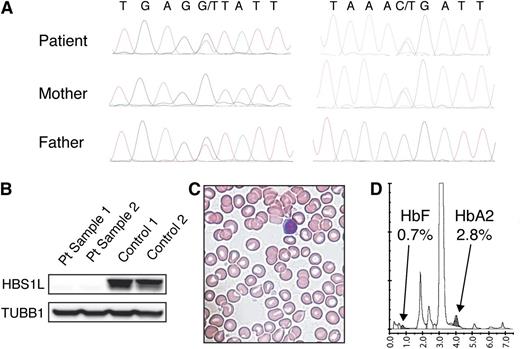
We have been screening children who have rare uncharacterized disorders using whole-exome sequencing. Approval for this study was obtained from the Boston Children's Hospital institutional review board. Informed consent was provided according to the Declaration of Helsinki. We identified a female child with a rare complete loss of function of the HBS1L gene. The patient was 3 years old at the time of the last clinical assessment, and her findings include severe intrauterine growth restriction, a 2-vessel umbilical cord with velamentous cord insertion, deep set eyes with blue sclerae, micrognathia, a bifid uvula with a submucous cleft palate, velopharyngeal insufficiency, sparse scalp hairs and eyebrows, microcephaly, global developmental delay, hypotonia, lax joints, fused C2-C3 vertebrae, scoliosis, and a left-sided diverticulum of the bladder with vesico-ureteral reflux. She had inherited a nonsense allele (chr6:135290431, c.1843C>T:p.R615X) from the mother and a null splice donor mutation (chr6:135287466, c.2043+1G>T) from the father in the HBS1L gene that we could confirm using Sanger sequencing (Figure 1A). Both of the mutations were extremely rare, with the splice site mutation present in only 1/13 005 alleles and the nonsense mutation not present in the the National Heart, Lung, and Blood Institute Exome Sequencing Project database (http://evs.gs.washington.edu/EVS/). Neither mutation was present in the 1000 Genomes Project database (http://www.1000genomes.org/). These null mutations should affect all major isoforms of the HBS1L gene. Consistent with this, we observed that the HBS1L protein was undetectable in fibroblasts from this patient (Figure 1B). A complete blood count that was performed on this patient was entirely within normal limits for age (white blood cells of 10 130/μL, hemoglobin of 12.4 g/dL, a red blood cell volume of 83.7 fL, and platelets of 372 000/μL), and a blood smear showed no abnormalities (Figure 1C). Hemoglobin electrophoresis was performed and showed a normal distribution of hemoglobin subtypes for age without an elevation of HbF or hemoglobin A2 (Figure 1D). Therefore, this rare complete loss of function in a patient strongly supports the notion that HBS1L is unlikely to mediate the effects of the association with hematopoietic traits that are seen on chromosome 6q23, which is also consistent with prior findings suggesting that the MYB gene may be responsible for this association using rare variants2 and functional studies.9
Complete loss of function of HBS1L results in a unique phenotype without hematologic abnormalities. (A) Sanger sequencing confirms the presence of compound heterozygosity for 2 loss-of-function mutations in HBS1L inherited from the father and mother, respectively. (B) Western blotting demonstrates the absence of HBS1L protein in 2 samples of fibroblasts from the proband, whereas it is present in 2 control samples. A tubulin β (TUBB1) loading control is present in similar amounts in all 4 samples. (C) A representative image of a blood smear shown at 100× shows normal red cell morphology without any other notable abnormalities, consistent with what is seen from examination of hundreds of similar fields. (D) Hemoglobin high-performance liquid chromatography analysis shows a normal distribution of hemoglobin subtypes in the patient.
Complete loss of function of HBS1L results in a unique phenotype without hematologic abnormalities. (A) Sanger sequencing confirms the presence of compound heterozygosity for 2 loss-of-function mutations in HBS1L inherited from the father and mother, respectively. (B) Western blotting demonstrates the absence of HBS1L protein in 2 samples of fibroblasts from the proband, whereas it is present in 2 control samples. A tubulin β (TUBB1) loading control is present in similar amounts in all 4 samples. (C) A representative image of a blood smear shown at 100× shows normal red cell morphology without any other notable abnormalities, consistent with what is seen from examination of hundreds of similar fields. (D) Hemoglobin high-performance liquid chromatography analysis shows a normal distribution of hemoglobin subtypes in the patient.
More generally, this observation shows how rare loss-of-function mutations can be extremely valuable for deciphering causal genes underlying specific traits or diseases identified through GWASs. The vast majority of such associations are found in noncoding regions, highlighting the utility of functional characterization of potential candidate genes underlying these associations using human loss-of-function alleles.1 With the increased output of exome sequencing projects, many more robust null alleles of a variety of genes are likely to be uncovered. As a result, it is important for large-scale sequencing projects to gather rich phenotypic information or have the ability to retrospectively ascertain clinical phenotypes of interest. This will help improve our understanding of both GWAS results and human gene function more generally, which will promote the development of more effective therapies for human disease.10
Authorship
Contribution: V.G.S. and P.B.A. designed the study; V.G.S., M.J., A.A., M.C.T., and P.B.A. performed the research; V.G.S., K.S.-A., N.M., K.M., G.T.B., and P.B.A. analyzed the data; and V.G.S. and P.B.A. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vijay G. Sankaran or Pankaj B. Agrawal, Boston Children's Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail sankaran@broadinstitute.org or pagrawal@enders.tch.harvard.edu.
References
Author notes
V.G.S. and M.J. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal