In this issue of Blood, Hahn and Lowrey have identified phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) as a novel mechanism for the post-transcriptional induction of fetal hemoglobin (HbF). Furthermore, they demonstrate that this eIF2αP-mediated pathway works synergistically with 2 clinical therapeutics, azacytidine and hydroxyurea (HU), to induce higher levels of HbF than any single agent alone.1
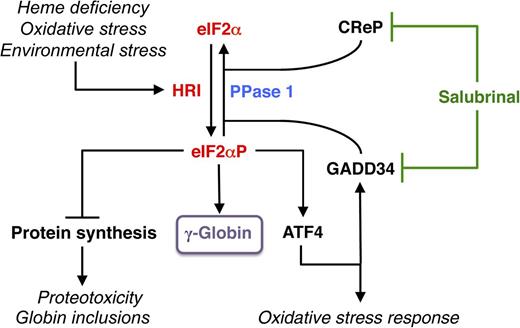
HRI-eIF2αP signaling in mitigating stress and induction of γ-globin expression. Among the family of eIF2α kinases, heme-regulated eIF2α kinase (HRI) is most predominant in the erythroid precursors. In heme deficiency, HRI is activated and phosphorylates eIF2α to balance globin synthesis with the intracellular heme concentrations ensuring that no globin is translated in excess of heme during erythroid maturation. HRI is also activated by oxidative stress and environmental stress, such as heat shock and osmotic shock. Under these stress conditions, the first action of eIF2αP is to inhibit general protein synthesis, especially globin to prevent proteotoxicity. Second, eIF2αP also increases translation of selective mRNAs, such as ATF4 mRNA to reprogram gene expression for adaptation to stress. GADD34 is a downstream target of ATF4 signaling, and it is responsible for bringing eIF2αP to type 1 phosphatase (PPase 1) for dephosphorylation to regenerate active eIF2, which is necessary for the recovery of protein synthesis of stress-induced gene expression that occurs late in the stress response. CReP has the same function as GADD34, except that it is constitutively expressed. Salubrinal, a selective inhibitor of eIF2αP dephosphorylation, interferes with the recruitment of eIF2αP to PPase1 through GADD34 and CReP, thus preventing eIF2αP dephosphorylation. Inhibiting eIF2αP dephosphorylation by salubrinal or by the knockdown of GAAD34 and CReP in differentiating human CD34+ cells induces γ-globin expression, leading to an increase in HbF production.
HRI-eIF2αP signaling in mitigating stress and induction of γ-globin expression. Among the family of eIF2α kinases, heme-regulated eIF2α kinase (HRI) is most predominant in the erythroid precursors. In heme deficiency, HRI is activated and phosphorylates eIF2α to balance globin synthesis with the intracellular heme concentrations ensuring that no globin is translated in excess of heme during erythroid maturation. HRI is also activated by oxidative stress and environmental stress, such as heat shock and osmotic shock. Under these stress conditions, the first action of eIF2αP is to inhibit general protein synthesis, especially globin to prevent proteotoxicity. Second, eIF2αP also increases translation of selective mRNAs, such as ATF4 mRNA to reprogram gene expression for adaptation to stress. GADD34 is a downstream target of ATF4 signaling, and it is responsible for bringing eIF2αP to type 1 phosphatase (PPase 1) for dephosphorylation to regenerate active eIF2, which is necessary for the recovery of protein synthesis of stress-induced gene expression that occurs late in the stress response. CReP has the same function as GADD34, except that it is constitutively expressed. Salubrinal, a selective inhibitor of eIF2αP dephosphorylation, interferes with the recruitment of eIF2αP to PPase1 through GADD34 and CReP, thus preventing eIF2αP dephosphorylation. Inhibiting eIF2αP dephosphorylation by salubrinal or by the knockdown of GAAD34 and CReP in differentiating human CD34+ cells induces γ-globin expression, leading to an increase in HbF production.
Patients afflicted with inherited β-hemoglobinopathies, principally sickle cell anemia and β-thalassemias, are born healthy due to predominant expression of fetal hemoglobin. Within 6 months, however, the switch from fetal to adult globin initiates the clinical onset of serious conditions manifested as intramedullary erythroid apoptosis, hemolytic anemia, and progressive organ damage. Reactivating endogenous γ-globin genes for enhancement of HbF production has become a translational holy grail in correcting both of these disorders.2 Clinical trials of therapeutics and HU responses suggest that achieving adequate HbF levels for many patients will require additional therapeutics with complimentary actions to promote HbF and restore red cell survival.2
Most therapeutics are known to induce γ-globin gene expression primarily at the level of transcription through histone acetylation and displacement of repressor complexes (butyrate), DNA methyltransferase-1 inhibition (decitabine), or transient cytostasis (HU). At certain doses, many HbF inducers also inhibit erythroid proliferation, which is counterproductive in anemias. Thalassemic erythroblasts suffer from oxidative stress and die at the polychromatophilic stage. As for sickle erythroblasts, sickle hemoglobin polymerization occurs early in differentiation. A concern in developing therapeutics is that rapid apoptosis in these conditions may preclude adequate HbF levels from being attained unless multiple approaches are found to act rapidly on fragile sickle and in stressed thalassemic erythroid progenitors. The report by Hahn and Lowrey1 indicates that these complex requirements are a step closer to achieving a therapeutic reality.
Phosphorylation of eIF2α elicits an integrated stress response under various stress conditions, and is conserved from yeast to humans. Elevated eIF2αP not only inhibits translation globally, but also specifically increases translation of certain messenger RNA (mRNAs) for subsequent transcriptional activation of stress response genes3 (see figure). This upregulation of translation requires the presence of upstream open reading frames in the 5′ untranslated region (UTR) of these unique mRNAs, most notably activating transcription factor 4 (ATF4) (see figure). In mammalian cells, four eIF2α kinases are expressed in distinct tissues to combat different stresses. PKR responds to viral infection while GCN2 senses nutrient starvations. PERK is activated by ER stress, and heme-regulated eIF2α kinase (HRI) is inhibited by heme.4
In the erythroid lineage, HRI is the predominant eIF2α kinase expressed at 2 orders of magnitude higher than the other 3 eIF2α kinases. In addition, HRI expression increases during erythroid differentiation with higher expression in the hemoglobinized erythroblasts, and contributes >90% of eIF2α phosphorylation.5 HRI is necessary to coordinate translation of globin mRNAs with the availability of heme for the production of large amounts of hemoglobin in red blood cells.4 Beyond heme deficiency, HRI is also activated by oxidative stress, osmotic shock, and heat shock. Recently, activation of the HRI-eIF2αP-ATF4 pathway has been demonstrated to mitigate oxidative stress and to promote erythroid differentiation in murine erythroid precursors.6
The steady state of eIF2αP in vivo is regulated by the equilibrium of eIF2α kinases and eIF2αP phosphatases. Salubrinal, a selective inhibitor of eIF2αP dephosphorylation, interferes with the recruitment of eIF2αP to PPase1 through growth arrest and DNA damage inducible protein 34 (GADD34) and constitutive repressor of eIF2α phosphorylation (CReP), thus preventing eIF2αP dephosphorylation7 (see figure). Hahn and Lowrey1 found that treatment of differentiating human CD34+ cells with salubrinal increases eIF2αP and shifts the polysome profile to monosomes, indicative of reduced translation. Most significantly, salubrinal increases HbF with a concomitant decrease of HbA without affecting cell proliferation, differentiation, or mRNA levels of γ- and β-globins. The role of eIF2αP in induction of HbF is further supported by 2 additional eIF2αP-modulating pharmacologic agents, BTdCPU (activating HRI) and guanabenz (affecting GADD34), as well as by knocking down GADD34 and CReP expression. Finally, they demonstrate that salubrinal can further increase HbF synthesis in cells treated with azacytidine or HU, establishing a different mechanism of action by salubrinal.
Although this study demonstrates eIF2αP induces HbF by a post-transcriptional mechanism, it remains to be determined whether this regulation occurs through γ-globin translation, or the stability of γ-globin protein. To the best of our knowledge, the only function of eIF2αP is to regulate translational initiation. In the future, it will be most important to investigate whether γ-globin mRNA is preferentially translated as compared with β-globin mRNA under conditions of elevated eIF2αP. If so, is this mediated by upstream open reading frames in the 5′UTR of γ-globin mRNA or by other mechanisms? It is also possible that a novel protein, which affects stability of γ-globin protein, is preferentially translated by elevated eIF2αP. Interestingly, earlier studies from the laboratories of Atweh8 and Lowrey9 also indicate the action of butyrate and azacytidine in enhancing γ-globin mRNA translation.
The action of salubrinal in erythroid cells may be more than through HbF induction. HRI is essential for reducing the phenotypic severity of β-thalassemia in mice, which do not express γ-globin. Salubrinal is effective in increasing eIF2αP, reducing denatured globins, and enhancing ATF4 translation in murine β-thalassemic erythroid cells, providing a strong basis for further exploitation of the HRI-eIF2αP signaling pathway to treat thalassemia.6 Although there is no known human disease associated with an HRI mutation to date, there is a positive association of 2 SNPs in the Ppp1r15a gene (encoding GADD34), with the induction of HbF by HU recently reported in sickle cell anemia patients.10 This study together with the article by Hahn and Lowrey underscore the critical role of HRI-eIF2αP signaling in human hemoglobinopathies.
Stimulation of HRI-eIF2αP signaling by salubrinal, and its additive effects with other drugs in inducing HbF, as demonstrated in this pivotal article by Hahn and Lowrey,1 provide evidence that the long sought definitive relief of the complex etiologies of anemia in the hemoglobinopathies is now on the horizon.
Conflict-of-interest disclosure: The authors declare no competing financial interests.