In this issue of Blood, Gay et al provide important new information that can be used to design future phase 3 trials.1
The key elements of modern treatment strategies: sequential treatment approach for myeloma. Ongoing treatment with an induction phase, followed by intensification (transplant), consolidation, and a maintenance phase.
The key elements of modern treatment strategies: sequential treatment approach for myeloma. Ongoing treatment with an induction phase, followed by intensification (transplant), consolidation, and a maintenance phase.
They chose a dose of melphalan known to be tolerated in this age group and augmented its activity by using cassettes of treatment using novel agents during induction, consolidation, and maintenance and evaluated its activity and tolerability in the 65- to 75-year-old age group. It is clear that it is well tolerated, with minimal early mortality and is associated with good response and survival data. It is possible to compare these outcomes with representative historical data sets including the Medical Research Council (MRC) study where the overall survival (OS) in the nontransplanted group for melphalan-prednisone vs cyclophosphamide-thalidomide-dexamethasone was 30.6 vs 33.2 months, respectively, and for patients in the transplanted group >64 years of age was 53 months.2 In the Intergroup Français du Myélome (IFM) 99-06 study comparing melphalan-prednisone with melphalan-thalidomide-prednisone and with autologous stem cell transplant (ASCT) with 100 mg/m2 of melphalan, the OS in each group was 33.2, 51.6, and 38.3 months, respectively.3 Thus, the 5-year OS of 63% in the current study is good, using this novel sequential approach to treatment, which should now be evaluated formally against current standard approaches for this age group.
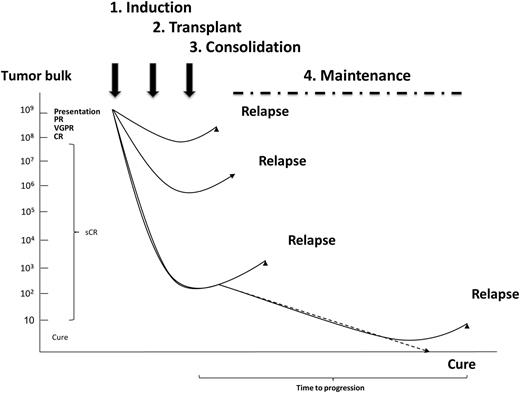
Maximizing cure rates by personalizing therapy is one of the major aims of modern myeloma treatment. With respect to achieving cure, one of the major prerequisites is the achievement of a stringent complete response (sCR) as assessed by multiparameter flow cytometry. It has been increasingly possible to achieve this aim in younger patients treated with ASCT where such sCRs are associated with optimum clinical outcomes.4 The integration of novel therapies into the context of ASCT has further improved overall and sCR rates and has changed the prevailing treatment paradigm which now comprises induction, transplant, consolidation, and maintenance treatment blocks. These blocks of treatment are delivered with the aim of sequentially reducing tumor bulk and incorporate the concept of using combinations of drugs incorporated into cassettes of treatment, which when combined have synergistic effects maximizing response rates. In addition, at each treatment phase, different cassettes comprising different combinations of drugs are given with the aim maximizing the chance of inducing apoptosis in resistant subclones and reducing the risk of relapse. More recently, the aim of maximizing responses has incorporated the concept of “maintenance treatment,” whereby ongoing treatment is given aiming to control the biology of residual clonal populations and killing myeloma stem cell populations coming into cell cycle.5,6
The incorporation of these ideas has revolutionized the treatment of younger patients, but it has come at the price of potentially increasing toxicity that needs to be managed. This is perhaps not an issue for the 40% of patients who develop myeloma before the age of 60, but for the majority of patients who develop myeloma beyond this age, toxicity is a significant issue. ASCT is considered the standard treatment of younger fitter patients, but there is no consensus in terms of an age cutoff above which ASCT is contraindicated. The initial IFM study used 60 years as an age cut point, whereas the MRC VII study used 65 years.7,8 In subsequent studies, including Myeloma IX, the MRC group used doctor and patient preference to decide on treatment pathway and included patients up to the age of 73 years in the transplant strategy. In an analysis of this study based on 5-year age cohorts, it was clear that there were differences in survival for the 55 to 60, 60 to 65, and 65 to 70 year groups, but mortality was not an issue. Interestingly, although a small number of patients over the age of 70 were included in the study, none made it to the transplant procedure for a number of reasons, including failure to collect stem cells and doctor or patient preference, making 70 a pragmatic age cutoff above which a full dose of 200 mg/m2 is generally inappropriate.2
The definition of an age cutoff of 70 years raises the issue of what is the best way of treating this age group, as well as younger patients who are considered too frail to receive a full dose of melphalan. One way of making high-dose melphalan more applicable is to reduce the dose. The standard dose of melphalan is 200 mg/m2, which reflects the fact that 3 positive studies comparing transplant to standard therapy used this dose,8 whereas studies using lower doses were not associated with positive survival benefits.3,9 In a variation on the single dose of melphalan approach, a sequential 100 mg/m2 approach has been evaluated and was found to be tolerable but again it did not improve survival.10
Conflict-of-interest disclosure: G.J.M. has participated in advisory boards for, received payment for lectures and development of educational presentations from, and received travel support from Celgene, Millenium, Novartis, Merck, and Johnson & Johnson.