In this issue of Blood, Ghosh and colleagues report that knocking out an inositol kinase in mice diminishes polyphosphate in platelet dense granules, thereby reducing hemostasis and protecting against thrombosis.1
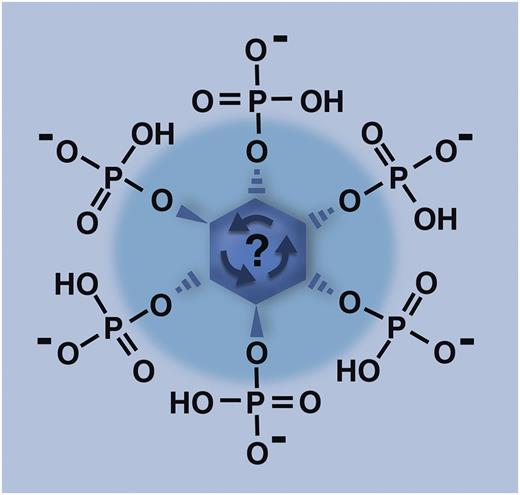
Inositol with all 6 hydroxyl groups phosphorylated (the molecule depicted here, termed inositol hexakisphosphate or IP6) is the substrate for inositol hexakisphosphate kinase 1 (IP6K1), which adds yet another phosphate group to this molecule in order to create inositol pyrophosphates. In a manner not well understood, knocking out the IP6K1 enzyme in mice severely decreases the accumulation of polyphosphate in platelet dense granules, with consequences for platelet function in both hemostasis and thrombosis. Professional illustration by Marie Dauenheimer.
Inositol with all 6 hydroxyl groups phosphorylated (the molecule depicted here, termed inositol hexakisphosphate or IP6) is the substrate for inositol hexakisphosphate kinase 1 (IP6K1), which adds yet another phosphate group to this molecule in order to create inositol pyrophosphates. In a manner not well understood, knocking out the IP6K1 enzyme in mice severely decreases the accumulation of polyphosphate in platelet dense granules, with consequences for platelet function in both hemostasis and thrombosis. Professional illustration by Marie Dauenheimer.
In 2004, Ruiz et al first reported that the dense granules of human platelets contain abundant levels of polyphosphate (a simple linear polymer of inorganic orthophosphate), which is secreted upon platelet activation.2 In 2006, we showed that polyphosphate of the size secreted by activated platelets is a potent prohemostatic agent, capable of accelerating plasma clotting and substantially delaying fibrinolysis.3 In the ensuing years, our laboratory and others have further delineated the contributions of polyphosphate to hemostasis and thrombosis, showing in particular that long-chain polyphosphate (of the size that accumulates in microorganisms) is an extremely potent trigger of the contact pathway of clotting, while platelet-sized polyphosphate greatly accelerates downstream clotting reactions, enhances fibrin clot structure, and promotes the feedback activation of factor XI by thrombin.4 Polyphosphate is both procoagulant and proinflammatory in vivo,5 and certain cationic polyphosphate inhibitors have recently provided proof of principle that antagonizing polyphosphate’s procoagulant action in vivo protects against thrombosis in mouse models of both arterial and venous thrombosis.6 However, when I present our findings on polyphosphate, I am almost invariably asked about the possibility of knocking out polyphosphate accumulation in the dense granules of platelets in transgenic mice, as a way to study polyphosphate function in living animals. My stock answer is that, because no one has identified the enzyme or enzymes responsible for synthesizing polyphosphate in higher eukaryotes, we have no idea what gene to target to achieve this goal. But that has just changed.
For many years, it has been appreciated that the myo-inositol moiety of the membrane phospholipid, phosphatidylinositol, can be multiply phosphorylated. Phospholipase C can then release the phosphorylated headgroup of this lipid into the cytoplasm, yielding inositol phosphates like inositol 1,4,5-trisphosphate (IP3), which function in Ca2+ release and signal transduction cascades.7 More recently, it was discovered that myo-inositol can be much more heavily phosphorylated in cells, including versions of inositol in which all six –OH groups contain phosphate (inositol hexakisphosphate, or IP6, as depicted in the figure), or even more highly phosphorylated forms, termed inositol pyrophosphates (reviewed by Wilson et al8 ). In these latter molecules, at least one of the –OH groups contains pyrophosphate while the rest contain monophosphate (yielding IP7 or IP8, with 7 or 8 attached phosphates, respectively). Many of the enzymes responsible for synthesizing inositol pyrophosphates use IP6 (see figure) as the substrate, including, in mammals, 3 isozymes of the enzyme, inositol hexakisphosphate kinase (IP6K). The IP6K1 isozyme has been knocked out in mice, resulting in lower body weight, reduced insulin levels/insulin hypersensitivity, and defective spermatogenesis.9
Ghosh et al1 cleverly noted that yeast lacking the enzyme equivalent to IP6K are not only depleted of inositol pyrophosphates but that they also, perhaps surprisingly, have severely reduced levels of polyphosphate.10 Reasoning that a metabolic link between inositol pyrophosphates and polyphosphate may also exist in mammals, they explored the use of IP6K1 knockout mice as a means of generating animals with low levels of polyphosphate in their platelets. In the present study,1 they now report that, while the platelets of homozygous IP6K1 knockout mice have normal-appearing dense granules, these platelets have a roughly 3-fold reduction in polyphosphate levels. Furthermore, in in vitro studies, they report that the platelets from these animals exhibited slower platelet aggregation, supported lengthened clotting times when platelet releasates were added to plasma, and exhibited lower incorporation of polyphosphate into fibrin clots (with concomitantly altered clot ultrastructure that could be rescued by exogenous polyphosphate), compared with studies performed using platelets from wild-type littermates. In vivo, they demonstrated longer tail bleeding times and greater resistance to thromboembolism in the homozygous IP6K1 knockout mice compared with wild-type littermates. Because inositol pyrophosphates play roles in energy metabolism in yeast, Ghosh et al took pains to demonstrate that the levels of adenosine 5′-diphosphate (ADP) and adenosine triphosphate (ATP) in platelets from their knockout mice were normal, so the reduced platelet-mediated functions in these animals were not simply attributed to decreased secretion of ADP or ATP.
Taken together, the results from this study clearly underscore important roles for polyphosphate secreted from activated platelets in both hemostasis and thrombosis. The decreased aggregation seen in platelets from homozygous IP6K1 knockout mice is particularly intriguing because a role in platelet aggregation has not previously been reported for polyphosphate. The availability of this mouse line for studying the consequences of severely reduced polyphosphate levels in platelets will allow a wealth of studies to investigate the biological roles of polyphosphates in mammals, no doubt beyond their currently appreciated contributions to hemostasis, thrombosis, and inflammation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal