Key Points
Inositol hexakisphosphate kinase 1 (IP6K1) knockout mice display lower inorganic polyphosphate levels in platelets.
Low platelet polyphosphate leads to lengthened clotting time, altered clot architecture, and protection against pulmonary thromboembolism.
Abstract
Polyphosphate (polyP), a polymer of orthophosphate moieties released from the dense granules of activated platelets, is a procoagulant agent. Inositol pyrophosphates, another group of phosphate-rich molecules, consist of mono- and diphosphates substituted on an inositol ring. Diphosphoinositol pentakisphosphate (IP7), the most abundant inositol pyrophosphate, is synthesized on phosphorylation of inositol hexakisphosphate (IP6) by IP6 kinases, of which there are 3 mammalian isoforms (IP6K1/2/3) and a single yeast isoform. Yeast lacking IP6 kinase are devoid of polyP, suggesting a role for IP6 kinase in maintaining polyP levels. We theorized that the molecular link between IP6 kinase and polyP is conserved in mammals and investigated whether polyP-dependent platelet function is altered in IP6K1 knockout (Ip6k1−/−) mice. We observe a significant reduction in platelet polyP levels in Ip6k1−/− mice, along with slower platelet aggregation and lengthened plasma clotting time. Incorporation of polyP into fibrin clots was reduced in Ip6k1−/− mice, thereby altering clot ultrastructure, which was rescued on the addition of exogenous polyP. In vivo assays revealed longer tail bleeding time and resistance to thromboembolism in Ip6k1−/− mice. Taken together, our data suggest a novel role for IP6K1 in regulation of mammalian hemostasis via its control of platelet polyP levels.
Introduction
Inorganic polyphosphate (polyP) consists of linear polymers of orthophosphate moieties linked by phosphoanhydride bonds. This high energy anionic molecule is found in all organisms, from archaea to mammals, varying in chain length and physiological function.1 PolyP of chain length of 60 to 100 phosphate units is present in dense granules of mammalian platelets at a concentration of ∼130 mM (expressed in terms of phosphate monomers).2 Platelet polyP regulates the blood clotting cascade at various points in the extrinsic, intrinsic, and common pathways.3 PolyP released from platelet dense granules initiates clotting via the contact pathway by acting as an anionic surface to trigger factor XII cleavage.4,5 Activation of factor XI by thrombin and autoactivation by factor XIa is accelerated in the presence of platelet length polyP.3,6 At the beginning of the common pathway, polyP accelerates the conversion of factor V to Va by both thrombin and factor Xa.7 As activated factor Va converts prothrombin to thrombin, platelet-derived polyP causes a localized thrombin burst at the clot site. The rapid formation of factor Va antagonizes the anticoagulant activity of tissue factor pathway inhibitor.7,8 An additional procoagulant function of polyP downstream of thrombin generation is the incorporation of polyP into fibrin clots, resulting in increased fibrin fiber thickness and altered fibrin distribution within the clot.9,10 Fibrin clots containing polyP dissolve more slowly compared with clots formed in the absence of polyP.9,10 The presence of polyP in the clot attenuates fibrinolysis by blocking the binding of tissue type plasminogen activator or plasminogen to fibrin. PolyP also slows fibrinolysis as a consequence of increased thrombin generation by enhancing the activity of thrombin-activatable fibrinolysis inhibitor.7 Platelet activation by extracellular histones is potentiated in the presence of polyP.11 It was recently shown that platelet α-granules also contain polyP at 500-fold lower levels than dense granules.12 PolyP in α-granules is bound to von Willebrand factor (vWF) and enhances vWF-dependent platelet aggregation. PolyP also acts as a proinflammatory agent, as demonstrated by stimulation of bradykinin generation in human plasma by synthetic polyP4 and by polyP derived from acid-rich granules of mast cells.13
In budding yeast, polyP accumulates predominantly in vacuoles and is synthesized from ATP by the vacuolar membrane transporter chaperone (VTC) complex.14 Yeast polyP levels are dependent on the presence of another family of high-energy phosphate-rich small molecules called inositol pyrophosphates.15,16 These consist of an inositol ring substituted with monophosphate and diphosphate groups. The inositol pyrophosphate 5-diphosphoinositol pentakisphosphate (5-IP7) is synthesized from inositol hexakisphosphate (IP6) by IP6 kinases (IP6Ks). The VIP1 family of enzymes converts 5-IP7 to 1,5 bis-diphosphoinositol tetrakisphosphate (IP8).17,18 In an alternate pathway, VIP1 converts IP6 to 1-IP7, which is then phosphorylated by IP6Ks to generate IP8.19 Saccharomyces cerevisiae lacking the IP6 kinase KCS1 have undetectable levels of inositol pyrophosphates and substantially reduced levels of polyP.20,21 It is therefore believed that the cellular levels of inositol pyrophosphates and polyP are metabolically linked.22
Mammals have 3 isoforms of IP6 kinases, IP6K1, IP6K2, and IP6K3,23,24 and 2 isoforms of VIP1.25,26 Ip6k1 knockout (Ip6k1−/−) mice display lower body weight, reduced insulin levels, and defective spermatogenesis compared with wild-type (WT) littermates.27 Mouse embryonic fibroblasts (MEFs) isolated from Ip6k1−/− embryos have 70% reduced IP7 levels compared with WT MEFs. To determine whether the link between inositol pyrophosphate and polyP levels is conserved in mammals, we measured platelet polyP levels in Ip6k1−/− mice. We observe a significant reduction in platelet polyP concomitant with altered clotting parameters and clot architecture in these mice. In vivo studies indicate that the absence of IP6K1 and subsequent decrease in platelet polyP reduces the susceptibility of these animals to pulmonary thromboembolism. Our study therefore highlights a novel role for mammalian IP6K1, acting upstream of polyP, and thereby indirectly regulating hemostasis.
Methods
All reagents, unless otherwise stated, were procured from Sigma-Aldrich (St. Louis, MO). All procedures were at room temperature unless specified otherwise.
Animals
The Ip6k1 gene knockout mouse was generated as previously described27 and back-crossed into the C57BL/6 strain for 7 generations. Ip6k1 heterozygous mice were bred to generate littermate WT and Ip6k1−/− mice. All mice used in this study were 8- to 20-week-old age-matched males, unless specified otherwise. They were housed in the animal facility at the Centre for Cellular and Molecular Biology, Hyderabad, or the Centre for DNA Fingerprinting and Diagnostics animal facility located within the premises of Vimta Labs, Hyderabad. Animals were maintained as per guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forests, Government of India. All animal experiments were approved by the Institutional Animal Ethics Committees of the respective animal facilities.
Platelet preparation
Mice were anesthetized by inhalation of an isofurane:oxygen (3:97) mixture or by an intraperitoneal injection of ketamine (20 mg/kg body weight) and xylazine (20 mg/kg body weight) and subjected to retro-orbital blood sampling. Three hundred microliters of blood was collected into 3.2% tri-sodium citrate (blood to anticoagulant ratio 9:1). Platelet-rich plasma (PRP), obtained by differential centrifugation of the citrated blood at 200g for 2 minutes followed by 100g for 2 minutes, was directly used in some assays. Washed platelets were prepared by centrifuging the PRP at 2500g for 4 minutes in the presence of prostaglandin I2 (PGI2, 500 nM; Calbiochem, Billerica, MA) to prevent platelet activation. Platelets were resuspended in 50 µL modified N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–Tyrode’s buffer (140 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, and 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 6.7). In some cases, platelets were pelleted in the absence of PGI2, and the supernatant was used as platelet-poor plasma (PPP).
IP6K1 detection
IP6K1 expression in platelets and megakaryocytes was monitored as described in supplemental Methods on the Blood website.
Platelet polyP detection
PolyP in platelet acidic extracts was quantified by measuring orthophosphate (Pi) released on treatment with the S cerevisiae exopolyphosphatase PPX1, as described in supplemental Methods.
Functional analysis of platelets
Thrombin-stimulated P-selectin surface expression, adenosine 5'-diphosphate (ADP)-stimulated surface expression of integrin αIIbβ3, and platelet serotonin levels were measured by flow cytometry. Platelet ultrastructure was examined by transmission electron microscopy. Platelet aggregation was determined spectrophotometrically by monitoring an increase in transmission. These procedures are described in detail in supplemental Methods.
Clot turbidimetry
Washed platelets prepared in the absence of PGI2 were activated with ADP (200 µM) at 37°C for 30 minutes and centrifuged at 18 000g for 15 minutes at 4°C. Platelet releasates in the supernatant were mixed with an equal volume of the corresponding PPP in 96-well flat bottom plates. Clotting was induced by recalcification with CaCl2 (5 mM) in a final reaction volume of 150 µL, and the change in turbidity was monitored at 405 nm in a microplate reader (SpectraMax Plus384; Molecular Devices, Sunnyvale, CA).
Clot ultrastructure
Platelet releasates were prepared as described in the Clot turbidimetry section. PPP was supplemented with 5% Alexa Fluor 488–conjugated fibrinogen (Invitrogen, Carlsbad, CA). Fifty microliters of this PPP was mixed with 50 µL platelet releasate and 4′,6-diamidino-2-phenylindole (DAPI; 40 µM) in a coverglass bottom dish. Clotting was initiated by recalcification (CaCl2, 5 mM) in a final volume of 150 µL and incubated in a dark humidified chamber for 30 minutes. Clot architecture was imaged by confocal fluorescence microscopy (LSM 510 META; Carl Zeiss, Jena, Germany) with a 63×/1.4 NA oil objective (multiphoton laser Ex760 nm, and Em535-590 nm for DAPI; argon laser Ex488 nm, and Em500-550 nm for Alexa Fluor 488). Four different fields were imaged for each clot, and ImageJ software (Analyze Particles plugin) was used to determine fiber density, which is the number of fibers crossing a 100-µm line averaged over 3 different positions in each image. PolyP detection by DAPI staining is described in supplemental Methods. The relative fluorescence intensity of DAPI quantified using ImageJ reflected the amount of polyP entangled in the clot. In some cases, the PPP was supplemented with polyP45 (50 µM; Sigma-Aldrich) or IP7 (100 µM) prior to the addition of platelet releasate.
In vivo clotting analyses
Tail bleeding time and pulmonary thromboembolism were monitored by standard procedures, as described in supplemental Methods.
Data analysis
All data were plotted and analyzed using GraphPad Prism (La Jolla, CA). P < .05 was considered statistically significant.
Results
Reduced polyP levels in Ip6k1−/− platelets
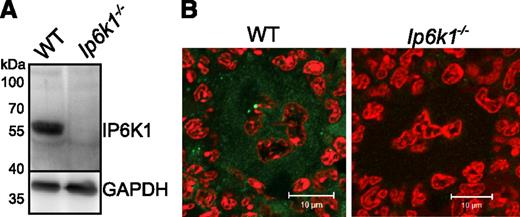
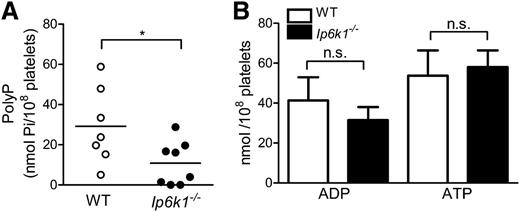
To investigate the link between inositol pyrophosphates and polyP in mammalian platelets, we first tested for the presence of IP6K1 in mouse platelets by immunoblotting with an IP6K1-specific antibody. We observe that extracts from WT mouse platelets contain IP6K1, but there is no detectable band in Ip6k1−/− platelets (Figure 1A). We also conducted immunofluorescence analysis of mouse bone marrow sections to look for the presence of IP6K1 in platelet progenitor megakaryocytes. Although IP6K1 was detectable in the cytoplasm of WT megakaryocytes, no immunostaining was observed in Ip6k1−/− bone marrow sections (Figure 1B). As MEFs derived from IP6K1 knockout embryos show a 70% reduction in intracellular IP7,27 it is likely that Ip6k1−/− platelets also have reduced amounts of inositol pyrophosphates. Because inositol pyrophosphate levels correlate with polyP levels in yeast,20,21 we examined platelets isolated from WT and Ip6k1−/− mice for the presence of polyP. Although WT platelets displayed a wide variation in levels of polyP, there was a significant overall reduction in polyP in Ip6k1−/− platelets (Figure 2A). In yeast, loss of the IP6 kinase KCS1 leads to increased ATP and reduced ADP levels.28 We therefore measured ADP and ATP levels in platelet extracts from WT and Ip6k1−/− mice and found no significant difference between the 2 sets of platelets (Figure 2B). These data show that the loss of IP6K1 in platelets leads to a reduction in polyP, as seen in yeast, but no change in ADP or ATP, both of which are important modulators of platelet function.
IP6K1 is expressed in platelets and megakaryocytes. (A) Western blot analysis of lysates prepared from WT and Ip6k1−/− platelets pooled from 3 mice of each genotype, using an antibody against IP6K1. GAPDH was used as a loading control. The blot is representative of 3 independent experiments. (B) Immunohistofluorescence analysis of IP6K1 expression (green) in femur bone marrow sections. Nuclei are counterstained with propidium iodide (red). Images were acquired by confocal microscopy (LSM 510 META; Carl Zeiss) using a 63×/1.4NA objective (Carl Zeiss). To improve visualization of IP6K1 staining (green), images were subjected to a 10% linear increase in brightness using LSM Browser (Carl Zeiss), and nonlinear adjustment of contrast and tonal range using Adobe Photoshop (level adjustment). Scale bars represent 10 µm.
IP6K1 is expressed in platelets and megakaryocytes. (A) Western blot analysis of lysates prepared from WT and Ip6k1−/− platelets pooled from 3 mice of each genotype, using an antibody against IP6K1. GAPDH was used as a loading control. The blot is representative of 3 independent experiments. (B) Immunohistofluorescence analysis of IP6K1 expression (green) in femur bone marrow sections. Nuclei are counterstained with propidium iodide (red). Images were acquired by confocal microscopy (LSM 510 META; Carl Zeiss) using a 63×/1.4NA objective (Carl Zeiss). To improve visualization of IP6K1 staining (green), images were subjected to a 10% linear increase in brightness using LSM Browser (Carl Zeiss), and nonlinear adjustment of contrast and tonal range using Adobe Photoshop (level adjustment). Scale bars represent 10 µm.
PolyP levels are reduced in Ip6k1−/− platelets. (A) Estimation of polyP levels in platelets isolated from WT and Ip6k1−/− mice, expressed in terms of phosphate monomers (Pi). Symbols represent each animal, and lines indicate the mean polyP level in each group. (B) Platelet ADP and ATP levels in WT and Ip6k1−/− mice. Data are mean ± standard error (n = 5 mice of each genotype). P values are from a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
PolyP levels are reduced in Ip6k1−/− platelets. (A) Estimation of polyP levels in platelets isolated from WT and Ip6k1−/− mice, expressed in terms of phosphate monomers (Pi). Symbols represent each animal, and lines indicate the mean polyP level in each group. (B) Platelet ADP and ATP levels in WT and Ip6k1−/− mice. Data are mean ± standard error (n = 5 mice of each genotype). P values are from a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
Analysis of platelet function
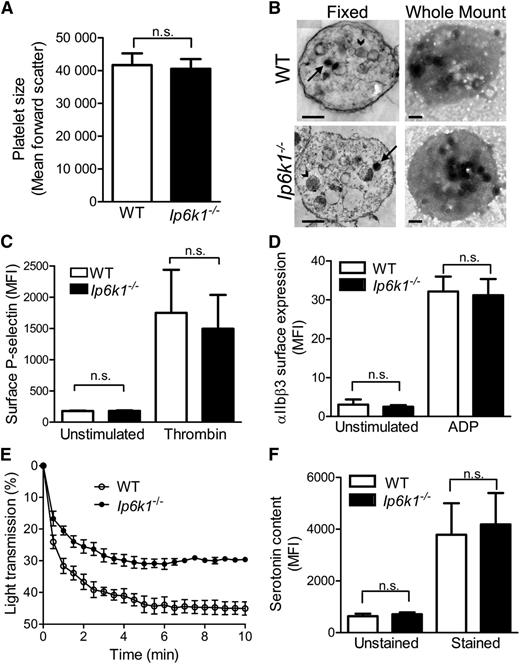
We wondered whether the reduction in polyP levels in Ip6k1−/− platelets results in an alteration of platelet characteristics in these mice. Analysis of hematologic parameters in WT and Ip6k1−/− mice revealed no difference in platelet count between these groups (Table 1). Other blood parameters, including red and white cell counts, hematocrit, and hemoglobin concentration, were also found to be normal in Ip6k1−/− mice. Platelet size, ascertained by flow cytometry measurement of forward scatter in washed and fixed platelets, was found to be comparable in WT and Ip6k1−/− mice (Figure 3A). We observed no morphological difference between WT and Ip6k1−/− platelets by transmission electron microscopy (Figure 3B). Whole mount transmission electron microscopy, which is routinely used to detect platelet dense granules, revealed no obvious alteration in the appearance and number of these structures in Ip6k1−/− platelets (Figure 3B).
Hematologic analysis
| Hematology parameters . | WT . | Ip6k1−/− . |
|---|---|---|
| Red blood cells, ×1012/L | 9.645 ± 0.3416 | 9.482 ± 0.2545 |
| White blood cells, ×109/L | 8.047 ± 0.8731 | 8.565 ± 0.8824 |
| Platelets, ×109/L | 1164 ± 97.40 | 1108 ± 59.12 |
| Hemoglobin, g/dL | 14.02 ± 0.3038 | 14.02 ± 0.2315 |
| Hematocrit, percentage | 47.22 ± 1.073 | 47.08 ± 0.6725 |
| Hematology parameters . | WT . | Ip6k1−/− . |
|---|---|---|
| Red blood cells, ×1012/L | 9.645 ± 0.3416 | 9.482 ± 0.2545 |
| White blood cells, ×109/L | 8.047 ± 0.8731 | 8.565 ± 0.8824 |
| Platelets, ×109/L | 1164 ± 97.40 | 1108 ± 59.12 |
| Hemoglobin, g/dL | 14.02 ± 0.3038 | 14.02 ± 0.2315 |
| Hematocrit, percentage | 47.22 ± 1.073 | 47.08 ± 0.6725 |
Characterization of Ip6k1−/− platelets. (A) Flow cytometry analysis of fixed platelets from WT and Ip6k1−/− mice to determine forward scatter as a reflection of platelet size. Data are mean ± standard error (n = 5). (B) Representative (left) fixed and (right) whole mount transmission electron micrographs of platelets pooled from 3 mice of each genotype. In fixed samples, arrows indicate dense granules and arrowheads indicate α-granules. Dense granules are clearly visible in whole mount micrographs. Bars represent 0.5 µm. (C) Surface P-selectin expression in unstimulated and thrombin stimulated WT and Ip6k1−/− platelets analyzed by flow cytometry. Data are mean ± standard error (n = 3). (D) Binding of Alexa Fluor 488–conjugated fibrinogen to integrin αIIbβ3 on the surface of unstimulated and ADP-stimulated WT and Ip6k1−/− platelets, monitored by flow cytometry. Data are mean ± standard error (n = 5). (E) Thrombin stimulated aggregation of washed platelets from WT and Ip6k1−/− mice measured spectrophotometrically as a decrease in percent light transmission over a period of 10 minutes. Samples were pooled from 3 mice of each genotype for the analysis. Data are mean ± standard error from 3 independent experiments. (F) Flow cytometry analysis of washed and fixed WT and Ip6k1−/− platelets labeled with a serotonin antibody, followed by an Alexa Fluor 488–conjugated secondary antibody (stained) or the secondary antibody alone (unstained). Data are mean ± standard error (n = 6). Data were analyzed by a 2-tailed Student t test (n.s., not significant, P > .05). MFI, median fluorescence intensity.
Characterization of Ip6k1−/− platelets. (A) Flow cytometry analysis of fixed platelets from WT and Ip6k1−/− mice to determine forward scatter as a reflection of platelet size. Data are mean ± standard error (n = 5). (B) Representative (left) fixed and (right) whole mount transmission electron micrographs of platelets pooled from 3 mice of each genotype. In fixed samples, arrows indicate dense granules and arrowheads indicate α-granules. Dense granules are clearly visible in whole mount micrographs. Bars represent 0.5 µm. (C) Surface P-selectin expression in unstimulated and thrombin stimulated WT and Ip6k1−/− platelets analyzed by flow cytometry. Data are mean ± standard error (n = 3). (D) Binding of Alexa Fluor 488–conjugated fibrinogen to integrin αIIbβ3 on the surface of unstimulated and ADP-stimulated WT and Ip6k1−/− platelets, monitored by flow cytometry. Data are mean ± standard error (n = 5). (E) Thrombin stimulated aggregation of washed platelets from WT and Ip6k1−/− mice measured spectrophotometrically as a decrease in percent light transmission over a period of 10 minutes. Samples were pooled from 3 mice of each genotype for the analysis. Data are mean ± standard error from 3 independent experiments. (F) Flow cytometry analysis of washed and fixed WT and Ip6k1−/− platelets labeled with a serotonin antibody, followed by an Alexa Fluor 488–conjugated secondary antibody (stained) or the secondary antibody alone (unstained). Data are mean ± standard error (n = 6). Data were analyzed by a 2-tailed Student t test (n.s., not significant, P > .05). MFI, median fluorescence intensity.
We also monitored activation of WT and Ip6k1−/− platelets by 2 agonists: thrombin and ADP. Thrombin-mediated activation was examined by measuring the levels of surface P-selectin, a cell adhesion molecule released from secretory α-granules (Figure 3C). No alteration in P-selectin surface expression in Ip6k1−/− platelets implies that IP6K1 does not influence platelet α-granule content or its thrombin-stimulated release. ADP stimulation of platelets was tested by measuring binding of fluorescently labeled fibrinogen to washed platelets treated with ADP. Fibrinogen binding to platelet surface integrin αIIbβ3 reflects platelet activation and is critical for platelet aggregation. We observe no difference in the extent of fibrinogen binding to activated WT or Ip6k1−/− platelets (Figure 3D), indicating that both sets of platelets respond normally to ADP stimulation. Following activation by different agonists, platelets adhere and aggregate to form a plug at the site of injury, leading to primary hemostasis. Platelet aggregation on thrombin stimulation was measured in washed platelets isolated from WT or Ip6k1−/− mice. We observe a significant decrease in the extent of aggregation of Ip6k1−/− platelets (Figure 3E). Platelets aggregate using fibrinogen and vWF as connecting agents. Although fibrinogen binding to integrin αIIbβ3 is normal in Ip6k1−/− platelets, it is possible that vWF function is compromised due to lowered levels of polyP.12
Trafficking of cargo to dense granules is dependent on the adaptor protein complex AP3, the function of which is compromised in IP6K1 knockout MEFs.29 It is possible that the decrease in platelet polyP in Ip6k1−/− mice is secondary to an AP3-dependent trafficking defect. Serotonin is a major constituent of platelet dense granules, and defective AP3-mediated trafficking leads to a reduction in platelet serotonin levels.30 We therefore measured serotonin levels in platelets isolated from WT and Ip6k1−/− mice by flow cytometry analysis of fixed platelets. No difference in serotonin content was observed between WT and Ip6k1−/−-derived platelets (Figure 3F), implying that the polyP deficiency in Ip6k1−/− platelets is not due to defective AP3 mediated trafficking to dense granules, but most likely results from a direct metabolic link between IP7 and polyP.21,22
Altered clotting parameters in Ip6k1−/− mice
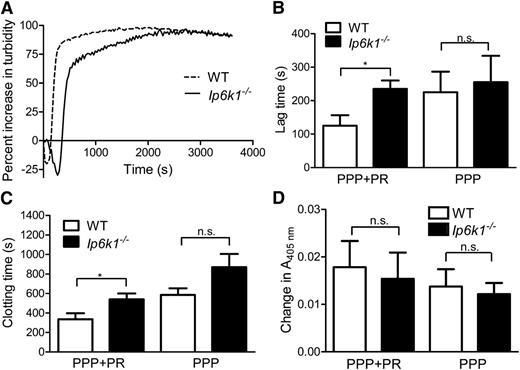
Our data thus far have established a molecular link between the presence of IP6K1 and polyP in platelets. PolyP influences plasma clotting upstream of thrombin generation.3 We therefore used ADP instead of thrombin to activate and degranulate washed platelets and generate releasates used in subsequent analyses to measure the influence of polyP on clotting. Platelet-length polyP have been shown to reduce clotting time on the addition of excess Ca2+ to citrated plasma in vitro.7,8 We slightly modified this assay by adding platelet releasates from WT and Ip6k1−/− mice to their autologous citrated PPP, prior to recalcification and clot turbidity measurement (Figure 4A). Following recalcification, plasma fibrinogen is converted to insoluble fibrin monomers, which polymerize to form longer protofibrils. These protofibrils aggregate laterally to form a fibrin clot, which is reflected as an increase in turbidity measured at 405 nm. We measured the lag time after recalcification at which an exponential increase in absorbance, reflecting protofibril aggregation, is observed.31 The end of this exponential phase, at which the absorbance reading begins to plateau, was measured as the clotting time. Both the lag time and total clotting time are significantly lengthened in Ip6k1−/− samples (Figure 4B-C). On the other hand, the lag time and clotting time of recalcified PPP alone were unaltered in Ip6k1−/− compared with WT. This suggests that changes in platelet-derived factor(s) are responsible for the prolonged lag and clotting time observed in Ip6k1−/− samples when platelet releasates are reconstituted with PPP. We observe no significant difference in turbidity between WT and Ip6k1−/− samples (Figure 4D), in concurrence with recent studies showing that platelet-length polyP polymers (<100mers) do not influence fibrin clot turbidity.8
Prolonged clotting time in Ip6k1−/− mice. Change in turbidity as a function of time monitored spectrophotometrically at 405 nm in recalcified platelet releasates (PR) mixed with PPP. (A) Representative turbidimetry curves are shown for WT and Ip6k1−/− samples. (B) Lag time, the time after recalcification at which an exponential increase in absorbance is observed, (C) clotting time, the time taken to reach the end of the exponential phase, (D) and the absolute change in absorbance between clotting time and lag time were measured in WT and Ip6k1−/− samples of PPP+PR or PPP alone. Data, mean ± standard error (n = 6), were analyzed by a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
Prolonged clotting time in Ip6k1−/− mice. Change in turbidity as a function of time monitored spectrophotometrically at 405 nm in recalcified platelet releasates (PR) mixed with PPP. (A) Representative turbidimetry curves are shown for WT and Ip6k1−/− samples. (B) Lag time, the time after recalcification at which an exponential increase in absorbance is observed, (C) clotting time, the time taken to reach the end of the exponential phase, (D) and the absolute change in absorbance between clotting time and lag time were measured in WT and Ip6k1−/− samples of PPP+PR or PPP alone. Data, mean ± standard error (n = 6), were analyzed by a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
Altered clot architecture in Ip6k1−/− mice
Prior studies have demonstrated that polyP binds to fibrinogen and soluble fibrin and is incorporated into the polymerized fibrin clot.9,10 The presence of polyP within a clot alters its architecture, reducing clot homogeneity and changing fibrin distribution within the clot.10 To examine whether the reduction in platelet polyP levels in Ip6k1−/− mice leads to altered clot architecture, we prepared clots by recalcifying a mixture of PPP and clarified platelet releasate, spiked with fluorescently labeled fibrinogen, and stained these clots with DAPI to detect polyP. Clots examined by confocal microscopy revealed a homogenous web-like clot architecture in Ip6k1−/− samples, whereas thicker fibrin fibrils and tight fibrin aggregates interspersed with large pores were observed in WT clots (Figure 5A). We quantified the extent of clot homogeneity by measuring the average fiber density and observed a significant increase in the number of fibers per unit length in Ip6k1−/−-derived clots compared with WT (Figure 5B). WT clots also stained positive for the presence of polyP, with maximal staining in the knots (Figure 5A), whereas there was a fourfold reduction in DAPI staining of Ip6k1−/−-derived clots (Figure 5C).
IP6K1 influences clot ultrastructure via polyP. (A) Confocal fluorescence micrographs of recalcified fibrin clots prepared from WT and Ip6k1−/− platelet releasates mixed with autologous PPP, in the absence or presence of exogenous polyP or IP7, as indicated. Fibrin fibers are visualized by incorporating Alexa Fluor 488–conjugated fibrinogen, and polyP is stained with DAPI. Scale bars represent 10 µm. (B) Fibrin fiber density in clots described in A was quantified using ImageJ software as described in the section Clot ultrastructure. Data are mean ± standard error (n = 5 for untreated and n = 3 for polyP containing clots). (C) PolyP content was estimated using ImageJ software by measuring relative fluorescence intensity (arbitrary units [AU]) in the DAPI channel over the entire field, averaged over 3 fields per clot. Data are mean ± standard error (n = 3). P values are from a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
IP6K1 influences clot ultrastructure via polyP. (A) Confocal fluorescence micrographs of recalcified fibrin clots prepared from WT and Ip6k1−/− platelet releasates mixed with autologous PPP, in the absence or presence of exogenous polyP or IP7, as indicated. Fibrin fibers are visualized by incorporating Alexa Fluor 488–conjugated fibrinogen, and polyP is stained with DAPI. Scale bars represent 10 µm. (B) Fibrin fiber density in clots described in A was quantified using ImageJ software as described in the section Clot ultrastructure. Data are mean ± standard error (n = 5 for untreated and n = 3 for polyP containing clots). (C) PolyP content was estimated using ImageJ software by measuring relative fluorescence intensity (arbitrary units [AU]) in the DAPI channel over the entire field, averaged over 3 fields per clot. Data are mean ± standard error (n = 3). P values are from a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).
This difference in clot architecture between polyP-containing and -deficient clots derived from mice reflects prior studies conducted in vitro, which reported a similar difference in clots formed using purified fibrinogen in the presence or absence of polyP.9,10 We therefore predicted that adding back polyP during clot formation in Ip6k1−/− samples would result in Ip6k1−/−-derived clots that resemble the architecture of WT clots. Indeed, incorporation of polyP (average chain length, 45) during clot formation in WT and Ip6k1−/− samples resulted in thickening of fibrils, an increased number of knots, and reduction in fiber density, thus eliminating the differences observed between WT and Ip6k1−/− clots (Figure 5A-B). It is possible that IP7 plays a direct role in clot structure formation and that the difference between WT and Ip6k1−/− clots arises directly due to a reduction in IP7 levels rather than lowered polyP. To address this possibility, we added IP7 during WT and Ip6k1−/− clot formation and noted a loss of fibrin aggregates and homogenous clot architecture in both samples (Figure 5A). The presence of IP7 therefore does not rescue the structural alteration of Ip6k1−/− clots, whereas adding polyP results in Ip6k1−/− clots that closely resemble the architecture of WT clots. These data indicate that the effect of inositol pyrophosphates on clotting is not direct but instead reflects the metabolic link between inositol pyrophosphates and polyP synthesis.
Hemostasis defects in Ip6k1−/− mice
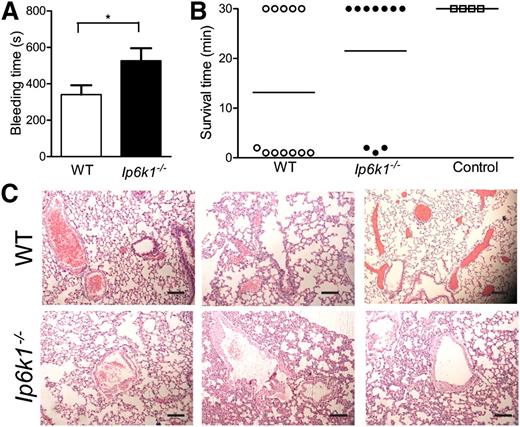
Platelets derived from patients with δ storage pool diseases (δSPDs), characterized by a defect in dense granule formation or function leading to bleeding diathesis, have substantially lowered levels of polyP compared with the general population.32 Delayed in vitro clot formation in such patient samples was shortened on addition of polyP.4 The loss of polyP has therefore been suggested to be a contributor to bleeding diathesis observed in δSPD patients. As Ip6k1−/− mice exhibit a similar decrease in platelet polyP, they too are likely to display bleeding diathesis. We therefore determined the bleeding time of WT and Ip6k1−/− mice by amputation of the tail tip. We note a significant lengthening of average bleeding time in Ip6k1−/− compared with WT mice (Figure 6A). Cessation of tail bleeding is primarily due to platelet plug formation as a result of platelet aggregation. The prolonged bleeding time in Ip6k1−/− mice reflects reduced aggregation of Ip6k1−/− platelets in vitro (Figure 3E) and concurs with the recent finding that cleavage of polyP bound to vWF leads to lower platelet aggregation.12 Our findings therefore support a role for polyP in primary hemostasis in the whole animal.
Altered hemostasis in Ip6k1−/− mice. (A) Bleeding time was measured following tail tip amputation in WT and Ip6k1−/− mice. Data are mean ± standard error (n = 11; *P ≤ .05). (B) Scatter plot indicating survival time of WT and Ip6k1−/− mice challenged with ADP to induce pulmonary thromboembolism. “Control” indicates WT mice injected with sterile water as a vehicle control. Animals that were alive 30 minutes after the challenge were considered survivors. (C) Hematoxylin and eosin–stained sections of lungs of WT and Ip6k1−/− mice that survived the challenge with ADP. Representative images from 3 different mice of each genotype are shown. Scale bars represent 100 µm.
Altered hemostasis in Ip6k1−/− mice. (A) Bleeding time was measured following tail tip amputation in WT and Ip6k1−/− mice. Data are mean ± standard error (n = 11; *P ≤ .05). (B) Scatter plot indicating survival time of WT and Ip6k1−/− mice challenged with ADP to induce pulmonary thromboembolism. “Control” indicates WT mice injected with sterile water as a vehicle control. Animals that were alive 30 minutes after the challenge were considered survivors. (C) Hematoxylin and eosin–stained sections of lungs of WT and Ip6k1−/− mice that survived the challenge with ADP. Representative images from 3 different mice of each genotype are shown. Scale bars represent 100 µm.
To examine the effect of reduced polyP on hemostasis in vivo, we used a pulmonary thrombosis model. Infusion of polyP into the vasculature has been shown to induce lethal pulmonary thromboembolism in mice.4 More recently, it was shown that mice treated with a cationic polymer that scavenges polyP are protected against lethal pulmonary thrombosis33 and display attenuated venous and arterial thrombosis.34 WT and Ip6k1−/− mice were challenged with a high dose of intravascular ADP to induce pulmonary thromboembolism, and animals were monitored for respiratory pattern and survival. Although fewer WT mice survived this challenge (Figure 6B), those that lived longer than 30 minutes exhibited signs of severe respiratory distress and were immobile. In contrast, the majority of Ip6k1−/− mice survived and were active following the challenge, displaying uniform breathing. Lung sections from WT and Ip6k1−/− mice that survived the challenge were examined for the presence of occluded vessels. We noted a higher degree of occlusion in large pulmonary vessels in WT compared with Ip6k1−/− mice, reflecting the difference in their breathing pattern (Figure 6C). Our results clearly indicate a role for IP6K1 in maintaining in vivo hemostasis by influencing platelet polyP levels.
Discussion
Our study identifies IP6K1 as a novel contributor to the maintenance of hemostasis in mammals. Specifically, we showed that platelets derived from mice lacking IP6K1 have reduced levels of polyP, resulting in longer clotting time and altered clot ultrastructure in vitro. Because we are able to restore the architecture of clots derived from Ip6k1−/− mice by adding polyP but not by adding IP7, we can infer that inositol pyrophosphates do not influence hemostasis directly but via polyP levels. As platelet polyP plays a major role in coagulation, Ip6k1−/− mice display longer bleeding time and are protected from in vivo thrombosis.
We demonstrated that platelets express IP6K1, and a recent proteomic analysis of human platelets documented the expression of VIP2,35 an enzyme that can convert 5-IP7 to IP8, implying that both inositol pyrophosphates, IP7 and IP8, are synthesized in platelets. The molecular mechanism linking the absence of IP6K1 to a reduction in platelet polyP is unclear. Evidence of a positive correlation between the levels of inositol pyrophosphates and polyP in yeast suggests that the same link may be conserved in mammals. S cerevisae lacking the IP6 kinase gene KCS1 exhibit dramatically reduced levels of inositol pyrophosphates and low to undetectable levels of polyP.20-22 When catalytically active but not inactive mouse IP6K1 was expressed in kcs1Δ yeast, polyP levels were restored, implying that inositol pyrophosphate synthesis by IP6 kinases is crucial to maintain the intracellular polyP pool.21 IP7 and IP8 may contribute to polyP synthesis or stability via a direct metabolic link22 or indirectly by pyrophosphorylating proteins involved in polyP synthesis or degradation.36,37 Inositol pyrophosphates have been shown to regulate phosphate uptake into cells,38 control intracellular levels of ATP,28 and are thought to be metabolic messengers that maintain cellular energy homeostasis.39 As polyP is a store of phosphate and energy, its synthesis is believed to be linked to phosphate entry and to ATP levels within the cell.40 However, we observe no changes in platelet ATP or ADP levels in Ip6k1−/− mice, suggesting that the influence of inositol pyrophosphates on platelet polyP is independent of adenine nucleotide levels. Although platelet polyP is not thought to be an energy store, our data suggest that the metabolic link between inositol pyrophosphates and polyP is evolutionarily conserved across all eukaryotes from yeast to mice, even in specialized cell types such as platelets.
The enzyme responsible for polyP synthesis in mammals has not yet been identified. In S cerevisae, subunit 4 of the VTC complex was identified a few years ago as a polyP synthase, but no paralogs of this enzyme exist in higher eukaryotes.14 The amoeba Dictyostelium discoideum, which has high levels of polyP, possesses 2 enzymes responsible for polyP synthesis: a homolog of the bacterial polyphosphate kinase, DdPPK1,41 and an actin-like polyP kinase, DdPPK2.42 Mammals do not possess genes homologous to the bacterial polyP kinases, but proteins similar to actin-like polyP kinases are present,43 and polyP synthesis in mammals is thought to be via an enzyme similar to DdPPK2. However, because our data show that the metabolic link between inositol pyrophosphates and polyP is conserved in yeast and mammals, it is possible that the mechanism for polyP synthesis in mammals is not dissimilar from that in yeast.
Although polyP was identified in platelet-dense granules almost a decade ago,2 and there have been significant advances in our understanding of its various effects on clotting,3 we are yet to fully understand the role of platelet polyP in an in vivo context. Studies conducted thus far have focused on understanding the effects of polyP on various clotting parameters such as clotting time, fiber density, factor V activation, factor XI activation, and abrogation of TFPI activity in isolated assays. Ip6k1−/− mice, which display altered hemostasis due to lowered platelet polyP levels, serve as a unique model to study the combined effects of polyP at multiple stages of the clotting cascade. Other functions in which polyP participates include cancer cell proliferation and metastasis,44-46 bone mineralization,47 and energy metabolism.48 Ip6k1−/− mice could also serve as a model to shed light on the mechanism and physiological significance of these and other functions of polyP.
Owing to its strong procoagulant properties, platelet polyP plays an important role in many clinical and pathological conditions. The clotting time of plasmas derived from patients of severe hemophilia A or B, or patients receiving the anticoagulant warfarin, was shortened by the addition of polyP.49 PolyP also reversed a defect in vWF-dependent platelet agglutination in patients of type I von Willebrand disease.12 Hermansky-Pudlak syndrome is a form of δSPD in which patients display dysfunctional biogenesis of lysosome-related organelles including platelet dense granules, leading to prolonged bleeding time in addition to other clinical features such as albinism. Lengthened plasma clotting time in Hermansky-Pudlak syndrome patients can be rescued by the addition of polyP.4 The prolonged bleeding time in Ip6k1−/− mice may reflect a novel form of δSPD, attributed only a specific loss of polyP, with no alteration in the levels of other dense granule components. Thromboembolism can lead to fatal pathological conditions including stroke, pulmonary embolism, deep vein thrombosis, and myocardial infarction. Our data demonstrating the resistance of Ip6k1−/− mice to ADP-induced pulmonary thromboembolism reveals that inositol pyrophosphates are key players in maintaining hemostasis. We speculate that specific inhibitors of IP6 kinases50 may function as antithrombotic agents. As IP6 kinases participate in several physiological functions, including insulin secretion, maintenance of body weight, and male fertility,27 further studies will be required to determine the pharmacological impact of reduced inositol pyrophosphate synthesis in the control of thrombosis.
Presented in abstract form at the Inositide Signalling in Health and Disease meeting, Coorg, India, November 29, 2012.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Solomon Snyder for the kind gift of Ip6k1+/− mice, Shashi Singh and M. Lakshman for conducting transmission electron microscopy, Adolfo Saiardi for sharing the pTrcHisB-PPX1 plasmid, Jomy Jose for processing histology samples, Joy Vallentyne, Jeddy Jose, Jayant Hole, Mahesh Kumar, and Vijay Pratap Singh for technical assistance, and Suhasini Kulkarni, L. Padmavathi, and all Laboratory of Cell Signalling personnel for valuable feedback.
This work was supported by core funds from the Centre for DNA Fingerprinting and Diagnostics, and Centre for Cellular and Molecular Biology, Hyderabad, India. D.S. was a recipient of a Department of Biotechnology Postdoctoral Fellowship (2009-2010). R.B. is supported by a Senior Fellowship from the Wellcome Trust/Department of Biotechnology India Alliance. R.B. would like to acknowledge the Department of Biotechnology, India (grants BT/PR11010/BRB/10/628/2008 and BT/BI/12/045/2008) for financial assistance.
Authorship
Contribution: S.G. performed most of the experiments; D.S. conducted the mouse tail bleeding analysis; K.S. and B.J.L. provided assistance for mouse experiments; R.M. synthesized and purified IP7 and PPX; S.K. provided valuable inputs and support; S.G. and R.B. designed the research and wrote the paper; and S.G., D.S., K.S., B.J.L., R.M., S.K., and R.B. read and agreed on the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.S. is Department of Biotechnology, GITAM Institute of Sciences, GITAM University, Rushikonda, Visakhapatnam, India.
Correspondence: Rashna Bhandari, Laboratory of Cell Signalling, Centre for DNA Fingerprinting and Diagnostics, Building 7, Gruhakalpa, 5-4-399/B, Nampally, Hyderabad 500001, India; e-mail: rashna@cdfd.org.in.

![Figure 5. IP6K1 influences clot ultrastructure via polyP. (A) Confocal fluorescence micrographs of recalcified fibrin clots prepared from WT and Ip6k1−/− platelet releasates mixed with autologous PPP, in the absence or presence of exogenous polyP or IP7, as indicated. Fibrin fibers are visualized by incorporating Alexa Fluor 488–conjugated fibrinogen, and polyP is stained with DAPI. Scale bars represent 10 µm. (B) Fibrin fiber density in clots described in A was quantified using ImageJ software as described in the section Clot ultrastructure. Data are mean ± standard error (n = 5 for untreated and n = 3 for polyP containing clots). (C) PolyP content was estimated using ImageJ software by measuring relative fluorescence intensity (arbitrary units [AU]) in the DAPI channel over the entire field, averaged over 3 fields per clot. Data are mean ± standard error (n = 3). P values are from a 2-tailed Student t test (*P ≤ .05; n.s., not significant, P > .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/8/10.1182_blood-2013-01-481549/4/m_1478f5.jpeg?Expires=1768263804&Signature=EuhTRVLEskqSF2PdLV8fqjKduHBOOYtdrKAIuSlyyzCmeqHodBzHx8nmlZJVw2uqkG0qwct58270cDWSj1OCEBsjBnm853KJiGgTxHb80tasQiwZxt1M0jpf2dSJUFf248t6YsO-JLrX5IwgCeGsvHYeZq4TmL~exLZHDLxEqAt4arZ9uI7BeejU-IGGb4bhCZhveOeP~k0hIA90UCjdX-XzYuxJNxqPHUh-GYqpW4iq0gIxdZc4mb2B-Il9yz4-Vf34WfqSDM67z87kP-zpYrinhfFhs6vX4sIbvn-emtdt~lmhuJwAi6znclLdJRgPVCVnM2~zdNADPi4Hiq9YXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal