Abstract
The treatment of older patients with acute lymphoblastic leukemia (ALL) is an unmet medical need. In Western countries, the population is aging, which means there will be an increasing number of older patients. However, in the past few decades, there has been little improvement in treating them, and few clinical trials specifically designed for older patients with ALL have been reported. Older patients with ALL have a significantly lower complete response rate, higher early mortality, higher relapse rate, and poorer survival compared with younger patients. This is partly explained by a higher incidence of poor prognostic factors. Most importantly, intensive chemotherapy with or without stem cell transplantation, both of which are successful in younger patients, is less well tolerated in older patients. For the future, the most promising approaches are optimized supportive care, targeted therapies, moderately intensified consolidation, and reduced-intensity stem cell transplantation. One of the most important challenges for physicians is to differentiate between fit and unfit older patients in order to offer both groups optimal treatment regarding toxicity and mortality risks, quality of life, and long-term outcome. Prospective trials for older patients with ALL are urgently needed.
Introduction
Acute lymphoblastic leukemia (ALL) is often perceived as a pediatric malignancy because the peak incidence occurs between 1 and 4 years of age.1 However, the incidence of ALL increases in the older population, and the proportion of patients diagnosed who are older than age 55 years (17%) nearly reaches that of patients age 21 to 54 years (22%).1 Because life expectancy of the general population is increasing, between 2010 and 2030, an increase of leukemia incidence of 67% is expected in patients older than age 65 in the United States.2
How should we define the term “old” in the context of leukemia treatment?
The term “young adults” is frequently used and was extended from age 35 to age 45 years; it is generally used for patients who tolerate and who appear to benefit from treatment with so-called pediatric-based chemotherapy.3 Does this mean that all patients not meeting the definition of “young” adult are “old” adults? My suggested approach is to define older patients on the basis of their assumed ability to receive intensive chemotherapy, including stem cell transplantation (SCT), which is usually limited to a cut point of 55 to 65 years. In this article, I will refer to patients beyond this age and discuss older patients as defined by biological features and not chronological age only.
Recruitment of older patients into clinical trials
In retrospective trials, the proportion of patients older than age 60 years in cohorts admitted to specialized centers varied from 18% to 30%.4-6 However, it is my personal impression that a considerable number of older ALL patients are not even referred; in addition, older patients are less frequently included in clinical trials. In a population-based study, the proportion of ALL patients included in clinical trials decreased from 74% in children to less than 10% in patients older than age 60 years.7 Trends are similar for trials with new drugs in cancer patients.8 This is because of age limits defined for clinical trials and the usual exclusion criteria, such as comorbidities or previous cancer.9 It may also be that older patients themselves are reluctant to be transferred to specialized centers and that physicians make less effort to provide comprehensive information to their patients. Furthermore, few studies are offered specifically for older patients. This is the domain of cooperative group trials, which are discouraged because of the increasing complexity of clinical trial legislation. Also as a result, studies on disease biology in older ALL patients are very limited, since biomaterial, usually collected in the context of cooperative group trials, is missing.
Biological and clinical features in older ALL patients
Biological features
The proportion of B-lineage ALL is higher in patients older (75% to 89%) than 60 years compared to patients younger (59% to 66%) than 60 years. Accordingly the incidence of T-ALL is lower in older (8% to 12%) compared to younger (29%) patients.6,10 A population-based study showed that cytogenetics were less frequently attempted in older (73%) compared with younger (85% to 91%) patients. The proportion of patients with Philadephia chromosome–positive (Ph+) t(9;22), t(8;14), t(14;18), or complex aberrations increased with age11 ; Ph+ ALL accounted for 24% to 36% in older patients vs 15% to 19% in younger patients.5,6 Considering the consequences resulting from diagnostic characterization, it should be self-evident that complete diagnostic characterization is required in all patients with ALL, regardless of age.
Clinical features
Features associated with large tumor mass or rapid progression, such as high white blood cell count, mediastinal tumors, or other organ involvement, appear to be less common in older patients.6,10 Even “smoldering” ALL is observed in some cases. Most studies report a lower proportion of males among older ALL patients.5,6 Secondary ALL after myelodysplastic syndromes or other malignant disease may become increasingly important, particularly in older patients; so far, very limited data are available.12 Performance status often deteriorates in older patients with onset of disease. In two studies, 30% to 43% of patients older than age 60 years vs 18% to 22% of younger patients had a performance status of 2 or more.5,6 Therefore, it is important to not only consider the current general condition in newly admitted older ALL patients but also to discern their status before the onset of leukemia-associated symptoms.
Comorbidity scoring
Of older ALL patients, 60% to 70% suffer from comorbidities13-15 but most studies did not refer to validated scoring systems. The German Multicentre Study Group for Adult ALL (GMALL) identified comorbidities according to the Charlson score in 84% of the patients older than 55 years, with diabetes (46%), vascular disease (18%), heart failure (15%), and chronic lung disease (12%) being the most frequent.13 In addition, renal insufficiency, anemia, osteoporosis, dementia, and depression are probably the most relevant comorbidities for potential adjustment of treatment. Eight percent to 16% had a history of prior malignant disease.6,13 I recommend a systematic evaluation and documentation of comorbidities based on a checklist or a score, since this is essential for planning an optimal treatment strategy.
CGA
Complete geriatric assessment (CGA) includes evaluation of daily living activities, comorbidities, depression, cognitive function, psychological status, nutrition, mobility, social situation, and other assessments16-18 and helps to identify unknown health problems and social factors. Furthermore, it may have a significant prognostic impact, as demonstrated in older lymphoma patients.19,20 In daily practice, CGA is often too time-consuming. Nevertheless, I suggest a systematic approach and, for practicability, use of a condensed patient self-report system,21-23 which could be part of institutional standard procedures for all older patients with hematologic malignancies.
Prognostic factors in older ALL patients
Increasing age itself is one of the most relevant prognostic factors for outcome of ALL from childhood to old age.5,15,24,25 Since older patients show opposite problems, namely higher mortality and relapse rates, prognostic factors for both have to be analyzed. Prognostic factors for relapse risk in younger ALL patients3,26 are probably also valid in older patients, such as early and mature T-ALL, pro-B ALL, elevated white blood cell count, and Ph+ ALL; however, their predictive value is somewhat diluted by mortality risks. Evaluation of minimal residual disease (MRD) has demonstrated that persistence of MRD is associated with a relapse rate above 90% in younger patients despite continued intensive chemotherapy.3,27 Few data on the prognostic impact of MRD are available in older patients. In one study, only 11% of the older patients with molecular failure after first consolidation remained in complete response (CR) compared with 68% of those with molecular remission.28 In older patients with less intensive therapy, a higher rate of MRD persistence and an even poorer outcome can be expected. Therefore, prospective evaluation of MRD in older patients is essential to identify those who could benefit from alternative experimental treatments, if they were available.
Several retrospective analyses in older patients with acute myeloblastic leukemia (AML) attempted to identify prognostic factors for the risk of early death.29,30 In ALL, bleeding and infection were associated with higher mortality.6,15 Body temperature, age, secondary AML, hemoglobin, platelet count, fibrinogen, and lactate dehydrogenase predicted a 7% to 63% early mortality risk in AML.31 It is not clear why some of these rather unspecific prognostic factors were relevant (eg, body temperature as an indicator of infections). It also remains unclear at which time point the factors should properly be assessed, whether outcome would have been different after the patients were stabilized (eg, by anti-infective treatment), and how performance status, comorbidity scoring, and geriatric assessment would fit in such models.
In the GMALL study for older patients, we identified comorbidity score, age, and performance status before onset of leukemia as prognostic factors with significant impact on early mortality.28 Interestingly, Eastern Cooperative Oncology Group (ECOG) status of 2 or more was documented in 7% of the patients before onset of leukemia-associated symptoms but in 38% after onset.28 The strong correlation of performance status with mortality was confirmed by others.5
For assessing prognosis in an older ALL patient, it is essential to identify features suitable for predicting high risk of early mortality resulting from complications. These features can help determine whether a patient has any chance of benefiting from intensive treatment. For this purpose, I would consider performance status before onset of leukemia, comorbidities, and geriatric assessment and would not rely on scores, which are calculated on the basis of historical patient cohorts.
In addition, prognostic factors for response to antileukemic treatment and relapse risk must be considered. Because of the lack of confirmed prognostic factors for older ALL patients, my approach would be to take known prognostic factors for younger patients into consideration but to focus on MRD evaluation as an individual prognostic feature that can cover the impact of biologic factors and also treatment intensity, compliance, and other unknown features.
General issues in management of older ALL patients
Co-medications
An estimated 78% of patients older than age 65 years take medications—a median number of five medications at admission—and up to 90% use over-the-counter drugs, including alternative therapies and dietary supplements.32,33 For example, many frequently used drugs either induce or inhibit the cytochrome P450 pathway, such as tyrosine kinase inhibitors (TKIs), cytostatic drugs, antidepressants, antibiotics, antifungals, and also herbal products such as St. John’s wort.
I would reconsider prescribed medications in detail, preferably by a systematic approach,34 to identify patients at risk and medications that should be avoided. The results of this analysis should be explained to the patient and the patient’s relatives to avoid unreported self-medications.
Risk of adverse events
In older patients, physiologic changes may have an impact on pharmacokinetics and pharmacodynamics of cytostatic drugs.35 In addition, specific drugs for treatment of ALL may be associated with a higher risk of toxicities, although it has never been demonstrated that comorbidities actually dispose for known toxicities of drugs (eg, anthracyclines and cardiac toxicities). Anticipated problems include polyneuropathies and constipation with vincristine, diabetes and hyperglycemia with steroid application, cardiac toxicities with anthracyclines, and liver toxicities induced by asparaginase, methotrexate, or purine analogs. Not all toxicities are well described, and recommendations for use specifically in older patients are not available. Scoring systems can help for prediction.36,37
I would like to emphasize that, despite concerns, the effective treatment of ALL as a life-threatening disease must be the primary focus. Physicians should keep in mind that in ALL, omission of effective drugs, dose reductions, and treatment delays are unfortunately associated with poorer outcome.
Induction therapy
There is no question that achieving CR is the prerequisite for long-term survival in ALL. Therefore, induction therapy is the most critical phase for management. In older patients, induction mortality has a wide range (0% to 42%) (Table 11,4-6,11,14,15,38-56 ). Seven percent to 10% of older patients die even before initiation of chemotherapy.6,15 The most frequent cause of death in induction is infection.38,39,57,58 Even with an age-adjusted chemotherapy, more than 95% of the patients experience grade 3 to 4 hematologic toxicity during induction.15,59 High rates of early mortality and of complications are also reported for palliative therapy approaches in older patients4 (Table 1). The biggest challenge of treating older ALL patients is limiting early mortality and, at the same time, avoiding reduction of treatment efficacy.
Supportive treatment
Taking the above mentioned risks into account, older patients require optimal supportive care. The application of granulocyte colony-stimulating factor during chemotherapy may somewhat attenuate neutropenia and influence infection-related mortality.60 Antibiotic prophylaxis is given in most centers, but the benefit of antifungal prophylaxis, particularly the use of azoles, has not been proven for ALL induction and it may contribute to additional toxicities, particularly those of vincristine.61
The question of whether antileukemic treatment has to be started immediately is important for practical management. At least one study showed that delays of treatment initiation were associated with poorer CR rates in younger but not in older patients.17,62 A recent retrospective analysis in AML patients confirmed that time from diagnosis to intensive chemotherapy did not influence the overall outcome.63
Certainly the need for action depends on the course of ALL in the individual patient. I think the approach of starting with a prephase treatment, collecting information on prognostic parameters, identifying potential therapy targets, initiating intensive supportive care, and giving the patient some time to accommodate to the situation is acceptable for many older patients and can help the patient arrive at the start of intensive chemotherapy in better condition. Obviously, unnecessary treatment delays should be avoided in all patients with ALL, regardless of age.
Treatment results in older ALL patients
Population-based studies
Registries give an impression of the overall outcome of unselected older ALL patients. In patients older than 65 years and older than 75 years, relative survival rates of 10% and 6%, respectively, were reported in the United States.1 In northern England ALL patients older than 60 years considered fit enough for active treatment had a survival of approximately 20% at 2 years and 12% at 5 years.11 The Swedish registry shows 2-year survival rates of 25% for ALL patients between age 65 and 74 years and 10% for those older than age 75 years.40
Palliative treatment compared with chemotherapy
Four groups have retrospectively compared results of palliative treatment with chemotherapy approaches. Thirty percent to 70% of the older patients were allocated to palliative therapy mainly because of poor performance status.4,5,11,41 Most studies showed an advantage of more intensive therapy such as higher CR rate, lower early death rate, remission duration, and median survival4,5,41 (Table 1). I would avoid the term “palliative treatment” in older patients with ALL since it is not well defined. Furthermore, it gives the wrong impression of good tolerability and quality of life, which have never been demonstrated in ALL patients.
Treatment according to protocols for adult ALL patients
The majority of published data are based on results reported for the subgroup of older patients treated within protocols designed for adult ALL patients in general. Most trials had no specific focus on older patients. Overall results for 519 patients older than 60 years can be extracted from these publications. The remission rate was 56%, with early death rates ranging from 6% to 42% (Table 1). I would assume that older patients included in protocols for younger patients often represent a selection of those in biologically good condition. In addition, non-predefined treatment modifications were often made and, altogether, potential conclusions from these studies are very limited.
Prospective studies for older ALL patients
Protocols specifically designed for older ALL patients have the theoretical aim of providing a chance of cure on one hand and of limiting toxicity, early mortality, and duration of hospitalization on the other and thereby maintaining as much quality of life as possible (Table 2).14,28,51-56,64
One central question is whether and which anthracycline needs to be included in induction regimens for older patients since these drugs contribute considerably to bone marrow toxicity. One approach is the use of idarubicine in induction therapy based on its supposed lower cardiac and hepatic toxicity. Idarubicine was combined with vincristine, prednisone, and asparaginase in induction and followed by a flexible outpatient postremission schedule. Induction mortality was 18%, and the CR rate was 59%. Survival was comparable to that in studies with more intensive therapy.51
A retrospective comparison of induction with liposomal daunorubicine at higher doses compared with a previously used conventional anthracycline-based regimen showed an improved remission rate (41% to 76%).14 When vincristine was replaced by vindesine in another study, no effect on the incidence of neurotoxicity was observed.52
A new induction regimen included continuous infusion with doxorubicine and vincristine randomly compared with pegylated doxorubicine and standard vincristine accompanied by dexamethasone and cyclophosphamide, followed by consolidation and maintenance. Despite lower rates of hematologic toxicity, infections, and cardiac events with pegylated doxorubicine, there was a trend toward a higher CR rate (72% vs 90%) and a lower rate of relapses (32% vs 52%) with conventional doxorubicin.56 Overall, the results of liposomal anthracyclines in elderly ALL are not convincing so far.
Asparaginase is an essential compound in the treatment of ALL. Little experience is available in older patients, and general experience in adult ALL shows that complications due to asparaginase are more frequently observed during induction compared with later treatment cycles. The PETHEMA group reported the results of an intensive induction regimen based on vincristine, daunorubicine, prednisone, cyclophosphamide, and asparaginase for elderly ALL. The induction mortality, mainly due to infection, was rather high at 36%. Therefore, cyclophosphamide and asparaginase were omitted from induction, resulting in a significant reduction of the early death rate from 70% to 22% and improved survival.53 A pediatric-based protocol used for older patients included asparaginase during induction and consolidation. The CR rate was 71% with 29% induction mortality and a number of complications, including infections (71%), cardiac toxicity (18%), and hyperglycemia (24%). Follow-up was short, with 71% survival and 82% relapse-free survival after 1 year.54 Altogether, there is some evidence that the use of asparaginase during induction therapy may be associated with increased risks. I would therefore advise against asparaginase in induction for older patients but would integrate this compound into consolidation therapy.
The majority of complications in older ALL patients is observed during induction, whereas there is still space for intensification of consolidation therapy. On the basis of this assumption, a consensus treatment protocol for older patients with ALL was defined by the European Working Group for Adult ALL (EWALL).65 The 4-week induction comprises dexamethasone, vincristine, and idarubicine in phase I and cyclophosphamide and cytarabine in phase II. Consolidation consists of 6 alternating cycles with intermediate-dose methotrexate combined with asparaginase and high-dose cytarabine followed by maintenance. The median age was 66 years (range, 56 to 73 years) with 22% of the patients being older than 70 years. The incidence of grade 3 to 4 cytopenias was 90%, and infections during phase I and II of induction occurred in 16% and 25% of the patients, respectively. Toxicities were less pronounced during consolidation, and asparaginase was well tolerated. CR, survival, and continuous CR rates after 1 year were 85%, 61%, and 49%, respectively.13 Although the early mortality rate was probably underestimated and follow-up was short, the moderate-intensity induction and consolidation treatment was tolerable. Recently an update on French data generated with the EWALL backbone was published. The major message was that an amendment with the use of asparaginase during induction led to major toxicities and mortality and that further intensification of consolidation appeared to be feasible.66
The GMALL conducted the largest prospective trial so far specifically designed for older patients. It was a Berlin-Frankfurt-Munster (BFM)-based dose-reduced induction therapy with idarubicine, dexamethasone, vincristine, cyclophosphamide, and cytarabine followed by alternating consolidation cycles for 1 year and maintenance similar to that in the EWALL regimen. Patients with CD20+ ALL received rituximab in combination with chemotherapy. In 268 patients, the CR rate was 76%, early death rate 14%, mortality in CR 6%, continuous remission 32%, and survival 23% at 5 years.28 Patients younger than age 75 years with an ECOG performance status below 2 had an 86% CR rate, 10% early death rate, and 36% survival at 3 years. Per amendment, we modified the induction therapy by the use of liposomal cytarabine for intrathecal prophylaxis instead of using a triple combination. This modification was associated with a reduction in early mortality, which may be explained by the fact that triple intrathecal therapy may, in contrast to liposomal cytarabine, contribute to systemic toxicities in induction. Furthermore, moderate intensification of consolidation with inclusion of high-dose cytarabine and methotrexate and native Escherichia coli asparaginase was tolerated. Overall, mortality in CR was only 6%.28
The superior results of pediatric-based regimens in ALL are undoubted. Therefore I am in favor of a pediatric-based approach for all patients who have ALL in all age groups. For older patients, the most important modification of induction therapy is probably omission of asparaginase and a flexible, reduced dose of anthracyclines as applied in the EWALL or GMALL protocols. In consolidation, intensified treatment should be attempted, and during this treatment phase, even asparaginase may be surprisingly well tolerated.
Treatment of older patients with Ph/BCR-ABL–positive (Ph+) ALL
The use of TKIs is a very promising approach for the large proportion of older patients with Ph+ ALL (Table 3).67-73 Nowadays, older patients with Ph+ ALL have an even better chance of achieving a CR than patients with Ph− ALL.
Even the application of imatinib in combination with chemotherapy after conventional induction can contribute to improved outcome of older patients with Ph+ ALL. Survival and relapse-free survival were significantly improved compared with a historical control without imatinib (66% vs 43% and 58% vs 11% at 1 year, respectively) in a French study.67 The use of TKIs upfront, however, is more promising. The GMALL conducted the first randomized study to evaluate the efficacy of imatinib single-drug induction compared with chemotherapy. Older patients with Ph+ ALL received 4 weeks of imatinib compared with chemotherapy induction. The remission rates were 96% and 50%, respectively. After induction, all patients received consolidation chemotherapy in parallel with imatinib for at least 1 year. In both arms, the relapse rate was high. Despite the significantly higher CR rate with imatinib induction, no difference in terms of survival was detected.68 Only 11% of the patients achieved a molecular remission. Recently, a follow-up was reported on a larger cohort of patients treated in a nonrandomized fashion according to similar regimens. The overall CR rate in 121 patients was 88%. Long-term survival at 5 years was 22%, and 19% remained in continuous remission at 5 years.69
The GIMEMA trial used imatinib (800 mg) with prednisone for induction followed by imatinib single-drug treatment. The remission rate, survival rate, and disease-free survival were 100%, 74%, and 48% after 1 year.70 A subsequent trial with dasatinib (140 mg) and prednisone followed by dasatinib single-drug treatment included patients with a median age of 54 years (range, 24 to 76 years). The CR rate was 92%, and survival was 69% at 20 months. Postremission therapy was at the discretion of the treating physician, and 14 of 19 patients with only TKIs relapsed with a high frequency of T315I mutations. Relapse risk was significantly influenced by molecular response.71 Another trial was based on a rotating schedule with 6 weeks of nilotinib treatment alternating with imatinib treatment. In 39 patients the remission rate was 94%, and the overall survival at 1 year was 79%. Nearly all relapsed patients in this trial showed mutations associated with TKI resistance.72
The largest prospective study so far in older patients with Ph+ ALL used the EWALL chemotherapy backbone with vincristine, dexamethasone, and dasatinib (140 mg) for induction. Consolidation and maintenance were combined with intermittent dasatinib applications. In 71 patients, the CR rate was 94%. The regimen was feasible, and the survival after 3 years of follow-up was 45%, which is promising. Persistent MRD above 0.1% after induction and consolidation was associated with a poorer remission duration of only 5 months. Dasatinib showed favorable antileukemic activity, but at relapse, a high frequency of T315I mutations was observed.73
Imatinib does not cross the blood-brain barrier. Therefore, central nervous system (CNS) prophylaxis is essential in Ph+ ALL. The number of intrathecal therapies and the type remain to be defined. Particularly in older patients, there could be an increased risk of subdural hematoma.74 Therefore intrathecal injections should be performed with specific precautions.75 It remains open whether less intensive CNS prophylaxis may be required if dasatinib, which crosses the blood-brain barrier, is used as first-line therapy.
Overall, single-drug treatment with TKIs can be used successfully for induction therapy in older patients with Ph+ ALL. The major advantage is the low early mortality rate. However, my expectation is that single-drug treatment in a disease as highly proliferative as ALL is per se associated with a high risk of selection of resistant clones. Therefore, after single-drug treatment with TKIs, most patients finally relapse if they are ineligible for SCT. Conversely, in frail older patients, TKI treatment offers the chance for prolonged survival with good quality of life. For older Ph+ ALL patients in good condition, I would nevertheless prefer TKIs in combination with a dose-reduced induction therapy followed by optimized consolidation. The EWALL trial provides some evidence that second-generation TKIs such as dasatinib or nilotinib may increase efficacy in this context. Identification of poor molecular responders and change of TKIs is essential. Furthermore, older patients with Ph+ ALL are candidates for reduced-intensity SCT. In MRD-negative older patients with Ph+ ALL, autologous SCT followed by maintenance with TKIs may be considered.
SCT in older patients with ALL
For patients older than age 55 to 65 years, the indication for SCT is rarely made because of the expected high transplant-related mortality (TRM), although reduced-intensity conditioning (RIC) might be promising. In selected older patient populations with a median age of 38 to 56 years, RIC yielded survival rates of 18% to 48%, relapse incidence rates of 36% to 50%, and TRM rates of 21% to 41%.76-81
In a recent large retrospective analysis with 127 patients with a median age of 56 years (range, 45 to 73 years) treated with RIC, the TRM was 17% with 51% survival. RIC was associated with a higher relapse risk, whereas full-intensity conditioning was associated with a considerable mortality up to 36% in patients older than age 60 years. With RIC transplantation, survival rates of 32% in those older than age 60 years were reported.79 Indication for SCT and the optimal conditioning regimen need to be defined. Furthermore, there is a dilemma since MRD is the most relevant prognostic factor for relapse risk in older patients, but outcome of SCT is poorer in MRD-positive ALL. Nevertheless, I would consider RIC-SCT in older patients with persistent MRD combined with an attempt to reduce MRD by targeted therapies, if they are available, and to measure MRD after SCT in order to administer either maintenance or immunologic therapies in case of MRD positivity.
Very limited data are available for SCT in older patients with Ph+ ALL. In one cohort, approximately 10% of the patients were transplanted in first CR. The median age was 62 years. Forty-eight percent of the patients with SCT survived compared with 22% of those without SCT.69 Although these data are very preliminary, they underline the need to further explore SCT in this patient group. The chance for effective RIC SCT procedures is probably better in Ph+ compared with Ph− ALL since maintenance with TKIs is a successful approach for controlling disease in combination with immunologic mechanisms.
New treatment options in older patients with ALL
ALL blasts express a number of antigens, such as CD33, CD22, CD19, and CD52, which could be targets for antibody therapy.82 The majority of older patients suffer from B-precursor ALL. In this subtype approximately half of the patients show CD20 expression on their blast cells. In younger patients with CD20+ ALL, the first promising data for the combination of chemotherapy and rituximab have been reported.83,84 Outcome of older patients could be hampered by a higher mortality due to infections in CR,84 which underlines the need for intensive supportive care for older patients throughout the entire treatment period.84
A promising new approach is the administration of a bispecific CD19 antibody, blinatumomab, which has the potential to engage cytotoxic T cells in patients for lysis of CD19+ leukemia cells.85 In 19 patients with refractory disease, defined as hematologic remission with persistent MRD after intensive chemotherapy, the molecular remission rate was 84%. A number of older patients who were not able to receive an SCT remained in remission for more than 1 year.86,87 More recently, a CR rate of 68% was reported for relapsed ALL. All patients with CR also achieved a molecular CR. Treatment with the final dosing regimen was well tolerated, and a number of older patients experienced a benefit.88 The CD22-directed, calecheamicin-conjugated antibody inotuzumab induced 18% CRs and 39% marrow CRs in relapsed CD22+ ALL. Toxicity appeared to be manageable, and the mortality of 4% within 4 weeks was moderate.89 Successful future use of antibody treatment will certainly depend on well-designed combination regimens with chemotherapy that aim to achieve long-term responses, particularly in older ALL patients.
Several other new drugs are of interest for optimizing treatment in older ALL patients. Although the number of older patients with T-ALL is low, the use of nelarabine is of interest after promising results and acceptable toxicity in relapsed T-ALL including older patients.90 Liposomal cytarabine for intrathecal application showed activity and tolerability in CNS relapse of ALL, although in combination with systemic neurotoxic regimens, severe toxicities may be observed.91 The use of liposomal cytarabine in prophylaxis of CNS relapse is of interest, particularly in older patients, since it allows reduction of the number of intrathecal injections and may induce fewer systemic toxicities compared with conventional intrathecal therapy.
Liposomal vincristine is another drug of interest, particularly in older patients. Results are still pending on the major question of whether liposomal encapsulation allows a higher dose-intensity with lower risk of neurotoxicity.92 Bendamustine could be of interest since it has shown limited toxicity and favorable results in older patients with B-cell lymphoma. New drugs with different mechanisms of action may, in the future, be used in combination with chemotherapy, such as proteasome inhibitors, histone-deacetylase inhibitors, hypomethylating agents, or targeted drugs such as Flt3 inhibitors or Jak2-inhibitors in defined subgroups of ALL.93 Currently, these compounds are either available in clinical trials or could be considered in individual patients with poor response to standard chemotherapy, including patients with molecular failure.
My recommendations for rational decision making for treatment of older adults
Older cancer patients are less likely to receive potentially curative treatment approaches because of physicians’ assumptions, justified or not, about disease-specific prognosis, general life expectancy, risk of complications, and their doubts regarding efficacy of treatment.94 Unfortunately, in some cases, even nonspecialists limit the treatment options offered to older patients by nonreferral to specialized centers.
In my opinion, it is essential to address one important misconception. Palliative, supportive treatment in acute leukemia does not, in general, reduce the risk of early death and does not improve quality of life compared with moderate intensive chemotherapy. Data from the Swedish registry show that in AML patients age 70 to 79 years with ECOG performance status above 2, the 8-week mortality was 76% for palliation compared with 50% for intensive treatment. In patients with ECOG performance status of 0 to 2, the corresponding rates were 23% to 47% for palliative treatment compared with 8% to 22% for intensive treatment. The variability depended on genetic risk.95,96 It is debatable whether treatment is actually justified in patients with mortality risks above 40% to 50%. However, a randomized trial in older AML patients confirmed not only a higher mortality and poorer survival but hospitalization times similar to those for a supportive care approach.97
The life expectancy has increased considerably in Western countries. Females at age 70 years have a life expectancy of 9.5 to 21 years, depending on their individual health status.18 As a result, in contrast to some solid tumors, in ALL, the risk of dying of the disease within an expected life period is, if not adequately treated, probably approaching 100%. This fact should be known when risks and chances of curative treatment are considered.
Although nowadays, a number of scores for risk of early mortality can be calculated by using the Internet, it should be kept in mind that they are based on historical data sets and that they are not validated for decision making. Although they may be helpful for informed consent, the result may unconsciously contribute to decisions against intensive treatment. I strongly emphasize that decision making is an individual process and the responsibility of the experienced physician; it should be based on a stepwise and rational assessment of all relevant individual factors and optimal communication with the patient (Table 4).
My suggestions for comprehensive management of older patients with ALL
Overall, the prognosis for older ALL patients is influenced by toxicities leading to higher mortality and to a higher rate of chemotherapy interruptions and reductions. This contributes, together with a higher incidence of poor prognostic features, to the higher relapse risk. How can this dilemma be resolved?
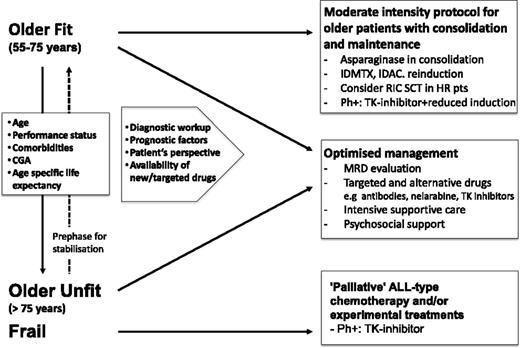
For general management, it is essential to distinguish between fit and unfit patients in whom an unacceptably high mortality from induction therapy has to be expected (Figure 1) based on a rational decision-making procedure (Table 4). A third group of patients are those with good general condition before onset of leukemia but have leukemia-associated complications; they may benefit from an extended prephase treatment with intensive supportive measures. For decision making, the patients’ perspectives, status, disease characteristics, and the expected outcomes have to be considered and discussed. It is essential to include relatives in informed consent procedures since they may become caregivers. Furthermore, decisions on nonintensive treatment may be misinterpreted by relatives and therefore should be explained in detail.
Comprehensive approach to managing older patients with ALL. HR, high risk; pts, patients; TK, tyrosine kinase; IDMTX, intermediate dose methotrexate; IDAC, intermediate dose cytarabine.
Comprehensive approach to managing older patients with ALL. HR, high risk; pts, patients; TK, tyrosine kinase; IDMTX, intermediate dose methotrexate; IDAC, intermediate dose cytarabine.
An attempt to achieve a remission should be made whenever possible. The major risk for older ALL patients during induction therapy is death resulting from infections. It is therefore essential to provide intensive supportive care, including anti-infectious prophylaxis and the use of granulocyte colony-stimulating factor. Conversely, any nonessential medication should be avoided to reduce the risk of cross-reactions and additional toxicities.
All older ALL patients need a comprehensive diagnostic classification, including at least immunophenotyping, molecular diagnostics, and setup of an assay for MRD evaluation. The identification of Ph+ ALL is crucial since, even in very old and frail patients, TKIs induce a high CR rate with reasonable durability. Furthermore, the biological characterization of older ALL patients needs to be improved. Biobanking for future scientific investigations within clinical trials should therefore be standard in older as it is in younger patients.
Altogether, in older as in younger patients, a pediatric-based induction strategy is recommendable in Ph− ALL. Dose reductions for anthracyclines are essential, and asparaginase during induction cannot be recommended outside of clinical trials. Dexamethasone appears to increase efficacy in younger patients but prolonged use should be avoided. For fit older patients, consolidation chemotherapy may be intensified. Moderate-dose consolidation, including methotrexate, cytarabine, and reinduction therapy appear to be feasible, and maintenance treatment is an essential treatment element.
In unfit older patients, a dose-reduced induction therapy is recommended with the aim of controlling and achieving a prolonged low-level disease. ALL-specific approaches should be considered, including vincristine, steroids, intrathecal therapy, and maintenance with mercaptopurine and methotrexate. Many physicians have more experience with older AML patients; however, there is no rationale for using AML regimens such as low-dose cytarabine or hydroxyurea in ALL.
When they are available, targeted drugs such as nelarabine, monoclonal antibodies, or other new drugs with potentially reduced or alternative toxicity should be added to treatment strategies in older patients, preferably in clinical trials. Since many of these compounds are used off-label, it may be useful to make the indication based on persistent MRD which, in addition, offers a chance to evaluate effects immediately. Treatment options may change as soon as new drugs or strategies become available. With effective drugs for prolonged maintenance, it may be possible to further reduce intensity of induction therapy and avoid early mortality in unfit patients.
In Ph+ ALL, it is still not clear whether further reduced induction chemotherapy adds an effect to TKI therapy and which inhibitor is preferable. I favor a combination therapy. Moderate dose consolidation and maintenance should be offered. Patients should be considered as candidates for RIC SCT.
Whereas full-conditioning regimens before SCT are clearly not recommended, RIC SCT is an option in older patients. For indication, it will be crucial to define prognostic factors. Because persistence of MRD is one of the most important risk factors, MRD evaluation should take place in older patients to identify those who could benefit from experimental therapies or SCT. This also applies to Ph+ ALL regarding the option of changing the TKI.
Management of older ALL patients is a real unmet medical need. A population-based study revealed that from 1980-1984 to 2000-2004, outcome of patients older than age 60 years remained nearly unchanged, with 8% vs 13% survival.98 Because of the lack of clinical trials and the reluctance to include older patients in trials, the gain of knowledge is slow and progress in terms of treatment optimization is limited. Prospective trials specifically designed for older ALL patients are needed, and patients should be entered onto trials or in registries whenever possible. Regulatory authorities should consider ways to make treatment optimization trials feasible in terms of bureaucratic burden and costs. Entry criteria, such as no longer excluding patients with prior malignancies, should be adapted. Structured documentation of comorbidity, limited CGA, as well as quality-of-life assessment should be standard in all trials for older ALL patients. Since ALL is a rare disease and a number of new drugs would be of interest, innovative trial designs with small randomized questions and short-term end points such as MRD should be favored.
Authorship
Contribution: N.G. wrote the manuscript.
Conflict-of-interest disclosure: N.G. has received research grants and honoraria for consultancy or speaker activities from Amgen, Bristol-Myers Squibb, EUSA Pharma, Genzyme, Gilead Sciences, GlaxoSmithKline, Mundipharma, Medac, Novartis, and Pfizer.
Correspondence: Nicola Gökbuget, Goethe University Hospital, Department of Medicine II, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: goekbuget@em.uni-frankfurt.de.