Abstract
Phosphatidylinositol and its phosphorylated derivatives, phosphoinositides, are minor constituents of phospholipids at the cellular membrane level. Nevertheless, phosphatidylinositol and phosphoinositides represent essential components of intracellular signaling that regulate diverse cellular processes, including platelet plug formation. Accumulating evidence indicates that the metabolism of phosphoinositides is temporally and spatially modulated by the opposing effects of specific phosphoinositide-metabolizing enzymes, including lipid kinases, lipid phosphatases, and phospholipases. Each of these enzymes generates a selective phosphoinositide or second messenger within precise cellular compartments. Intriguingly, phosphoinositide-metabolizing enzymes exist in different isoforms, which all produce the same phosphoinositide products. Recent studies using isoform-specific mouse models and chemical inhibitors have elucidated that the different isoforms of phosphoinositide-metabolizing enzymes have nonredundant functions and provide an additional layer of complexity to the temporo-spatial organization of intracellular signaling events. In this review, we will discuss recent advances in our understanding of phosphoinositide organization during platelet activation.
Introduction
Platelet plug formation is essential during hemostasis, but when perturbed, it can lead to pathological bleeding or thrombosis.1 Thus, this is a tightly controlled process requiring activation of platelets under carefully modulated intracellular signaling transduction.2,3 When there is a vascular injury, platelets tether to collagen or to von Willebrand factor and initiate an intracellular signaling cascade that leads to firm and stable adhesion to the subendothelium.4 This is followed by integrin activation on the platelet surface and, subsequently, aggregation between platelets.3 Further stabilization of the platelet plug and prevention of platelet disaggregation requires additional amplification of the platelet signaling pathways. Over the past few decades, accumulating evidence indicates that phosphorylated forms of phosphatidylinositol (PtdIns) are crucial components in this complex network of platelet signaling.
Phosphoinositide metabolism and signaling
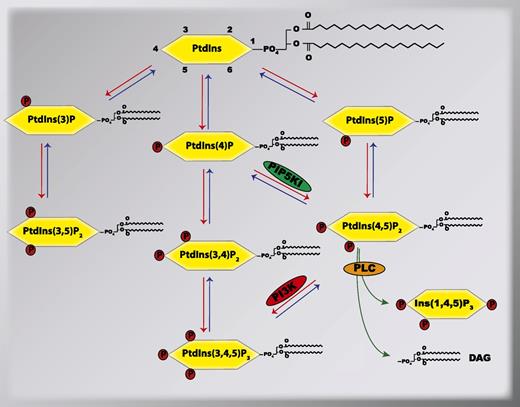
More than 50 years ago, PtdIns was initially observed to be modified by transient phosphorylation of its inositol head group, which generated various phosphorylated forms of PtdIns, currently known as phosphoinositides.5,6 Since that seminal observation, numerous studies have demonstrated that PtdIns and phosphoinositides are essential mediators of the signal transduction events that are involved in the regulation of diverse cellular processes, which include membrane trafficking, cytoskeletal dynamics, membrane transports, and nuclear events in many different cells.7 A total of 7 phosphoinositides have been identified that derive from PtdIns following the reversible phosphorylation of the hydroxyls situated in the D3, D4, and D5 positions of the inositol head group. These 7 phosphoinositides are PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, PtdIns(3,4)P2, PtdIns(4,5)P2, and PtdIns(3,4,5)P3 (Figure 1). These phosphoinositides are rapidly generated and degraded within distinct cellular compartments by specific phosphoinositide-metabolizing enzymes, which include lipid kinases, lipid phosphatases, and phospholipases.8,9 For instance, phosphatidylinositol-4-phosphate-5-kinase type I (PIP5KI) phosphorylates the PtdIns(4)P at the D5-OH group to generate PtdIns(4,5)P2 on the plasma membrane.10 In turn, PtdIns(4,5)P2 can be hydrolyzed either by a phosphatase converting it into PtdIns(4)P or by the phospholipase C (PLC) that selectively breaks it down into 2 second messengers, diacylglycerol (DAG) and inositol−1,4,5- trisphosphate [commonly known as either IP3 or Ins(1,4,5)P3].11 By their distinct expression and regulation patterns in different tissues, individual phosphoinositide-metabolizing enzymes contribute to the temporo-spatial organization of specific phosphoinositides in different cells. Once synthesized, the individual phosphoinositides can be recognized by selective subsets of proteins containing specific phosphoinositide-binding domains,12 thus providing each of the individual phosphoinositides with unique functions in cells.
Metabolism of phosphoinositides by phosphoinositide-metabolizing enzymes. Shown is the relationship between different phosphoinositides and their metabolizing lipid kinases (red arrows), lipid phosphatases (blue arrows), and PLC (green arrows). In this review, we focus on the signal transduction mediated by the lipid kinases PIP5KI and PI3K, and the PLC in platelets. PIP5KI, phosphatidylinositol-4-phosphate-5-kinase type I; PI3K, phosphatidylinositol-3-kinases; PLC, phospholipase C.
Metabolism of phosphoinositides by phosphoinositide-metabolizing enzymes. Shown is the relationship between different phosphoinositides and their metabolizing lipid kinases (red arrows), lipid phosphatases (blue arrows), and PLC (green arrows). In this review, we focus on the signal transduction mediated by the lipid kinases PIP5KI and PI3K, and the PLC in platelets. PIP5KI, phosphatidylinositol-4-phosphate-5-kinase type I; PI3K, phosphatidylinositol-3-kinases; PLC, phospholipase C.
The role of PtdIns(4,5)P2 signaling in platelet biology
PtdIns(4,5)P2 synthesis by PIP5KI
PtdIns(4,5)P2 is a predominant phosphoinositide in the cellular membrane. PtdIns(4,5)P2 is synthesized either from PtdIns(4)P by the D5-OH kinase activity of PIP5KI13 or from PtdIns(5)P by the D4-OH kinase activity of phosphatidylinositol-5-phosphate-4-kinase type II.10,14 Studies comparing the relative labeling rate of the D4- and D5-hydroxyl positions of the inositol ring suggest that the catalytic activity of the D5 position is more efficient.15,16 In addition, the relative abundance of PtdIns(4)P is much greater than PtdIns(5)P,16,17 suggesting that PIP5KI phosphorylation of PtdIns(4)P is the major source of PtdIns(4,5)P2 synthesis. In agreement with these findings, although the loss of phosphatidylinositol-5-phosphate-4-kinase type II does not reduce the synthesis of PtdIns(4,5)P2 in mammalian cells, the loss of PIP5KI significantly decreases the intracellular pool of PtdIns(4,5)P2. This demonstrates that PIP5KI is the predominant kinase in the generation of PtdIns(4,5)P2.
The role of PIP5KI isoforms and splice variants during platelet plug formation
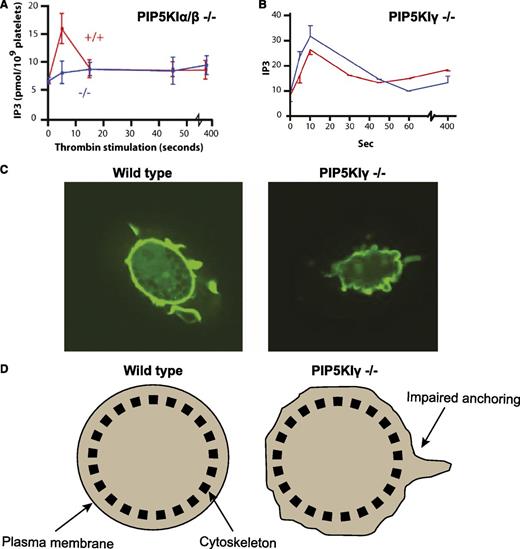
PIP5KI exists in 3 different isoforms (α, β, and γ). Intriguingly, all 3 PIP5KI isoforms can synthesize PtdIns(4,5)P2 from PtdIns(4)P.18-20 This in turn raises the question of why PIP5KI exists in more than 1 isoform. Recently, work by our group as well as by others addressed this question using genetically engineered mice lacking each isoform of PIP5KI in platelets. Platelets contain all 3 isoforms of PIP5KI, but the murine PIP5KI-β and PIP5KI-γ are the most abundant forms.21,22 Platelets lacking PIP5KI-β have defective synthesis of PtdIns(4,5)P2 as well as defective Ins(1,4,5)P3 formation in the first minute after stimulation with thrombin.22 Consequently, these platelets display defective aggregation ex vivo and impaired formation of an occluding thrombus in vivo.22 Remarkably, platelets lacking both PIP5KI-α and PIP5KI-β have a complete loss of Ins(1,4,5)P3 formation, even though they still contain PIP5KI-γ22 (Figure 2A). This suggests that PIP5KI-γ synthesizes a pool of PtdIns(4,5)P2 that is not required or sufficient for the synthesis of second messengers such as Ins(1,4,5)P3.
Isoform-specific functional roles of PIP5KI in megakaryocytes and platelets. (A) PIP5KI-α/β double knockout platelets have impaired generation of the second messenger Ins(1,4,5)P3 following activation with the platelet agonist, thrombin. (B) In contrast, PIP5KI-γ knockout platelets produce normal amounts of Ins(1,4,5)P3 after thrombin stimulation. (C) However, megakaryocytes differentiated from progenitor cells of PIP5KI-γ knockout mice display defective anchoring of their cellular membrane to their underlying cytoskeleton. This is shown in the confocal images of megakaryocytes bound to immobilized fibrinogen and stained with green fluorescence protein (GFP) fused to the PLC-δ pleckstrin homology domain. (D) Schematic cartoon illustrating defective plasma membrane attachment to the cytoskeleton in the PIP5KI-γ knockout megakaryocytes compared with the wild-type megakaryocytes when analyzed by an optical trap (laser tweezers).
Isoform-specific functional roles of PIP5KI in megakaryocytes and platelets. (A) PIP5KI-α/β double knockout platelets have impaired generation of the second messenger Ins(1,4,5)P3 following activation with the platelet agonist, thrombin. (B) In contrast, PIP5KI-γ knockout platelets produce normal amounts of Ins(1,4,5)P3 after thrombin stimulation. (C) However, megakaryocytes differentiated from progenitor cells of PIP5KI-γ knockout mice display defective anchoring of their cellular membrane to their underlying cytoskeleton. This is shown in the confocal images of megakaryocytes bound to immobilized fibrinogen and stained with green fluorescence protein (GFP) fused to the PLC-δ pleckstrin homology domain. (D) Schematic cartoon illustrating defective plasma membrane attachment to the cytoskeleton in the PIP5KI-γ knockout megakaryocytes compared with the wild-type megakaryocytes when analyzed by an optical trap (laser tweezers).
As predicted, platelets lacking PIP5KI-γ can still produce normal amounts of Ins(1,4,5)P3 upon stimulation with thrombin (Figure 2B), despite having impaired synthesis of PtdIns(4,5)P2.21 However, in contrast to cells lacking PIP5KI-α or PIP5KI-β, megakaryocytes or platelets lacking PIP5KI-γ have a significant defect in anchoring their cell membranes to the underlying cytoskeleton21,23 (Figure 2C-D). This suggests that PIP5KI-γ synthesis of PtdIns(4,5)P2 does not contribute to Ins(1,4,5)P3 formation, but rather directly affects the membrane anchoring of the cytoskeleton (Figure 3A). Additional evidence suggests that PIP5KI-γ synthesizes a discrete pool of PtdIns(4,5)P2 that interacts directly with actin-binding proteins (such as talin).24,25 Once bound to PtdIns(4,5)P2, these actin-regulating proteins are enabled to anchor the cell membrane to the underlying cytoskeleton.26
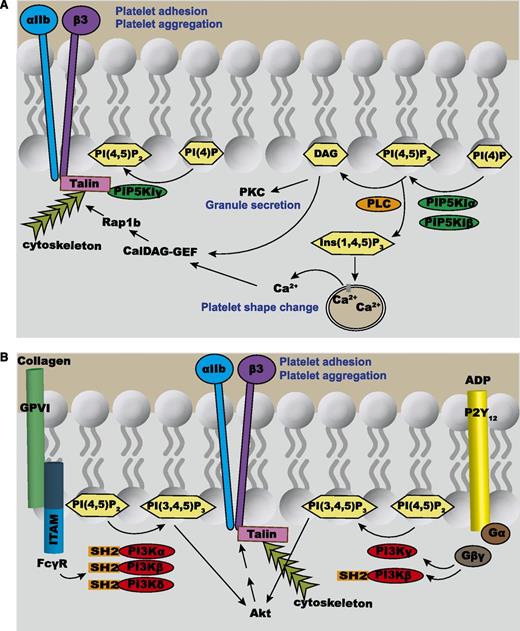
Overview of phosphoinositide signaling in platelets. (A) PIP5KI-α and PIP5KI-β synthesize the pool of PtdIns(4,5)P2 that is hydrolyzed by PLC-β (activated by thrombin and TxA2) or PLC-γ (activated by collagen) into the second messengers Ins(1,4,5)P3 and DAG. The Ins(1,4,5)P3 diffuses through the cytoplasm, binds to the Ins(1,4,5)P3 receptors on the DTS, thereby increasing the cytosolic concentration of Ca2+, which in turn activates multiple effector proteins. DAG is a second messenger that recruits to the plasma membrane protein kinase C (PKC). In platelets, membrane-bound PKC plays a crucial role in the secretion of granules. Both Ca2+ and DAG activate calcium- and diacylglycerol-regulated guanine nucleotide exchange factor (CalDAG-GEF), which then can activate a small GTPase, Rap1b. This enables Rap1b to activate the integrin αIIbβ3, a receptor that is crucial for platelet aggregation. In contrast to both PIP5KI-α and PIP5KI-β, PIP5KI-γ directly binds to talin, which helps link the integrins on the cell membrane to the underlying cytoskeleton. (B) Collagen binding to its receptor, GPVI, results in the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) within the FcRγ chains to enable the binding of the SH2 domains within PI3K-α, PI3K-β, and PI3K-δ. On the other hand, ADP binding to the Gi-coupled P2Y12 receptor triggers the release of Gβγ from the Gα subunit. Gβγ can then stimulate PI3K-γ and PI3K-β. All of the isoforms of PI3K are capable of synthesizing PtdIns(3,4,5)P3 by phosphorylating PtdIns(4,5)P2. PtdIns(3,4,5)P3 can bind to and activate several effector proteins including Akt. In turn, Akt activates αIIbβ3-mediated platelet aggregation.
Overview of phosphoinositide signaling in platelets. (A) PIP5KI-α and PIP5KI-β synthesize the pool of PtdIns(4,5)P2 that is hydrolyzed by PLC-β (activated by thrombin and TxA2) or PLC-γ (activated by collagen) into the second messengers Ins(1,4,5)P3 and DAG. The Ins(1,4,5)P3 diffuses through the cytoplasm, binds to the Ins(1,4,5)P3 receptors on the DTS, thereby increasing the cytosolic concentration of Ca2+, which in turn activates multiple effector proteins. DAG is a second messenger that recruits to the plasma membrane protein kinase C (PKC). In platelets, membrane-bound PKC plays a crucial role in the secretion of granules. Both Ca2+ and DAG activate calcium- and diacylglycerol-regulated guanine nucleotide exchange factor (CalDAG-GEF), which then can activate a small GTPase, Rap1b. This enables Rap1b to activate the integrin αIIbβ3, a receptor that is crucial for platelet aggregation. In contrast to both PIP5KI-α and PIP5KI-β, PIP5KI-γ directly binds to talin, which helps link the integrins on the cell membrane to the underlying cytoskeleton. (B) Collagen binding to its receptor, GPVI, results in the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) within the FcRγ chains to enable the binding of the SH2 domains within PI3K-α, PI3K-β, and PI3K-δ. On the other hand, ADP binding to the Gi-coupled P2Y12 receptor triggers the release of Gβγ from the Gα subunit. Gβγ can then stimulate PI3K-γ and PI3K-β. All of the isoforms of PI3K are capable of synthesizing PtdIns(3,4,5)P3 by phosphorylating PtdIns(4,5)P2. PtdIns(3,4,5)P3 can bind to and activate several effector proteins including Akt. In turn, Akt activates αIIbβ3-mediated platelet aggregation.
These studies demonstrate that although all PIP5KI isoforms can synthesize PtdIns(4,5)P2, they have nonredundant functional roles in platelets. How the different isoforms of PIP5KI promote unique physiological effects is unclear, but several mechanisms are proposed. First, although PIP5KI-α and PIP5KI-β have homologous primary sequences, PIP5KI-γ is structurally different.18,22 This suggests that individual PIP5KI isoforms could be regulated differently. Second, spatially distinct localization of individual PIP5KI isoforms may generate discrete pools of PtdIns(4,5)P2. Several studies indicate that different PIP5KI isoforms are localized in distinct cellular compartments in cells grown in culture. For instance, PIP5KI-α is more predominant in membrane ruffles,27 whereas PIP5KI-β concentrates close to the endosomes,28 and PIP5KI-γ is targeted to focal adhesions and nerve terminals.24,25,29 In addition, our data shown in Figure 2 indicate that PIP5KI-α and PIP5KI-β, but not PIP5KI-γ, can synthesize the pool of PtdIns(4,5)P2 used by PLC to generate Ins(1,4,5)P3.21,22 From these studies, one could hypothesize that the pools of PtdIns(4,5)P2 from different PIP5KI isoforms are highly compartmentalized and targeted by selective downstream effector molecules, providing unique functions to each of the PIP5KI isoforms. However, it is also conceivable that only PIP5KI-α and PIP5KI-β can interact with auxiliary proteins that in turn regulate the activity of downstream effectors such as PLC. Nevertheless, the exact mechanisms by which different PIP5KI isoforms fulfill unique niches within platelets need to be further elucidated.
As mentioned previously, several groups of researchers have speculated that PIP5KI-γ might generate PtdIns(4,5)P2 that binds to talin and positions it to participate in signaling pathways that emanate toward or in response to integrin activation.24-26,30 PIP5KI-γ has 2 predominant splice variants that differ by the inclusion of or exclusion of 27 amino acids encoded by the terminal exon of the PIP5KI-γ gene.24,25 These 2 splice variants are the longer 90-kDa (p90) splice form and the shorter 87-kDa (p87) splice form. These additional 27 amino acids allow the p90 splice variant to bind to talin, a protein that is important for both integrin activation and for linking integrins to the underlying cytoskeleton.24,25,30 Consequently, platelets and megakaryocytes lacking the p90 splice variant of PIP5KI-γ have defective anchoring of their integrins to the underlying cytoskeleton.23 This effect depends on the kinase activity of PIP5KI-γ, which implies that the local synthesis of PtdIns(4,5)P2 by the p90 splice variant plays an important role in maintaining the integrity of the connection between the cell membrane and actin. Remarkably, platelets lacking this p90 splice variant of PIP5KI-γ have no observable defect in the activation of integrins or in cell adhesion. In contrast, platelets lacking both p87 and p90 PIP5KI-γ do have a defect in shear-resistant adhesion.23 These data suggest that although both the p87 and p90 PIP5KI splice variants can synthesize PtdIns(4,5)P2, they have nonredundant functions in platelet cytoskeletal dynamics and in stable adhesion. This study further provides evidence that PIP5KI has a variety of intracellular functions. Not only does PIP5KI produce isolated pools of PtdIns(4,5)P2, but it also, because of its individual isoforms and splice variants, can directly interact and regulate distinct signaling proteins.
Regulation of PIP5KI by small guanosine triphosphatases (GTPases)
Several studies suggest that the localization and kinase activity of PIP5KI isoforms are regulated by small GTPases of the Rho family. For instance, Hartwig et al31 demonstrated that thrombin-mediated Rac1 activation induces the synthesis of PtdIns(4,5)P2 by PIP5KI-α, which in turn leads to actin filament assembly. The Offermanns group showed that exposure of platelets to thromboxane A2 (TxA2) stimulates Rho and Rho kinase, which thereby activate PIP5KI.32 We have shown that PIP5KI is rapidly translocated from the cytosol to actin-rich compartments upon platelet activation, but this process is impaired when the Rho GTPase is inhibited.33,34 Together, these works suggest that Rho family GTPases regulate the trafficking of PIP5KI into the membrane cytoskeleton. Once PIP5KI is positioned next to its substrate, PtdIns(4)P, PIP5KI is poised to generate PtdIns(4,5)P2.
The role of second messengers Ins(1,4,5)P3 and DAG in platelet biology
Generation of Ins(1,4,5)P3 and DAG by PLC
PLC hydrolyzes PtdIns(4,5)P2, thereby cleaving the bond between the glycerol and phosphate moieties to generate the second messengers DAG and IP3 (Figure 3A).35 PLCs are classified into 6 subfamilies based on sequence homology. They are PLC-β, PLC-γ, PLC-δ, PLC-ε, PLC-ζ, and PLC-η. Many of these PLC isoforms have multiple variants, leading to a very large and diverse family of enzymes.36 Although all PLC isoforms can hydrolyze PtdIns(4,5)P2 to generate DAG and Ins(1,4,5)P3, each PLC isoform has distinct cellular expressions, subcellular localizations, and specific modes of activation.37 This diversity further contributes to the temporo-spatial metabolism of PtdIns(4,5)P2 signaling.
The role of PLC isoforms in platelet functions
Human platelets contain different variants of PLC-β (PLC-β1, PLC-β2, PLC-β3, and PLC-β4)37,38 and PLC-γ (PLC-γ1 and PLC-γ2).39,40 PLC-β is activated when its pleckstrin homology domain binds to a Gβγ subunit of a heterotrimeric G-protein complex. This event occurs in platelets that are stimulated by soluble agonists such as TxA2 and thrombin (Figure 3A). In contrast, PLC-γ is activated when platelets are stimulated with an immobilized ligand. An example of this process occurs when collagen binds to the platelet’s glycoprotein VI (GPVI) receptor. This platelet collagen receptor is linked to Fc receptor-γ (FcR-γ) chains that contain immunoreceptor tyrosine-based activation motifs. Collagen binding to GPVI induces the FcR-γ chains to become phosphorylated, which then initiate a chain of signaling events that ultimately lead to the phosphorylation of PLC-γ and, subsequently, platelet adhesion41-43 (Figure 3A). Another example of a ligand that interacts with platelets and leads to activation of PLC-γ2 involves a C-type lectin receptor, CLEC-2.44,45 The platelet CLEC-2 binds to its ligand, podoplanin, which is expressed on the surface of other cells. This PLC-γ2–mediated signaling event plays an essential role in diverse processes including lymphangiogensis.45,46
Regardless of the activating mechanism, all PLC isoforms induce the rapid hydrolysis of PtdIns(4,5)P2 into Ins(1,4,5)P3 and DAG.36 Ins(1,4,5)P3 is a soluble second messenger that can diffuse through the cytosol and bind to Ins(1,4,5)P3 receptors,11 which in turn function as Ca2+ channels in the platelet dense tubular systems (DTSs). This triggers Ca2+ release from the DTS to the cytosol, which in turn modifies the cytosolic concentrations of Ca2+ and regulates diverse downstream signaling effects that include the release of platelet secretory granules47 (Figure 3A). In contrast to Ins(1,4,5)P3 generated by PLC-mediated hydrolysis of PtdIns(4,5)P2, the second messenger DAG simultaneously generated by this process functions as a lipid signaling molecule on cell membranes that is essential for the localization and activation of different isoforms of protein kinase C (PKC).48 In turn, PKC has regulatory functions in many cellular processes, which include proliferation, apoptosis, survival, and migration.48,49 In platelets, DAG-mediated activation of PKC also leads to the phosphorylation of a 47-kDa protein called pleckstrin, which is a protein that is important in platelet secretion.50
Patients who have a deficiency of PLC-β have a mild bleeding disorder.51,52 Ex vivo, platelets from these patients, or mice engineered to lack PLC-β isoforms, exhibit abnormal platelet aggregation and secretion.53 This is due to diminished PLC-β–mediated Ins(1,4,5)P3 production, intracellular Ca2+ mobilization, and pleckstrin phosphorylation.51-53 Although PLC-γ1 is expressed in several tissues, PLC-γ2 is primarily expressed in hematopoietic cells. Consistent with this tissue distribution pattern, PLC-γ1−/− mice are embryonic lethal,54 whereas mice genetically deficient in PLC-γ2 often exhibit subcutaneous hemorrhage during embryonic development, and surviving adults develop intraperitoneal and gastrointestinal hemorrhages.55 Although platelets lacking PLC-γ2 have normal response to thrombin or adenosine diphosphate (ADP), they exhibit defective aggregation ex vivo in response to collagen.42,55 In addition to its role in hemostasis, platelet PLC-γ2 has a critical role in lymphangiogenesis. This was demonstrated by a recent study showing a null mutation of the PLC-γ2 gene in a spontaneous mutant mouse line displaying blood-filled lymphatic vessels secondary to defective developmental separation of lymphatic and blood vessels.45 Collectively, these studies suggest that distinct isoforms of PLC carry out specific physiological roles in platelets as well as in different tissues.
The role of PtdIns(3,4,5)P3 signaling in platelet functions
PtdIns(3,4,5)P3 synthesis by PI3K
Phosphatidylinositol-3-kinases (PI3Ks) can phosphorylate the D3-OH group of the inositol ring within phosphoinositides to generate PtdIns(3,4,5)P3, PtdIns(3,4)P2, and PtdIns(3)P.56 There exist 3 classes of PI3K isoforms (I, II, and III).57 The class I PI3Ks are critical for the phosphorylation of PtdIns(4,5)P2 to generate the second messenger PtdIns(3,4,5)P3 (also known as PIP3).58 This class of PI3K is subdivided into class IA (PI3K-α, PI3K-β, and PI3K-δ) and IB (PI3K-γ). The class IA PI3K is a heterodimer that is composed of a catalytic subunit (p110-α, p110-β, and p110-δ) bound to a regulatory subunit (p85-α, p85-β, p55-γ, p55-α, and p50-α) that possesses an Src homology 2 (SH2) motif. Because SH2 domains bind to tyrosine-phosphorylated residues on other proteins, the regulatory subunit enables class IA PI3K to bind to tyrosine-phosphorylated receptor tyrosine kinases or to adaptor molecules.59 This process unfolds the PI3K complex and enables it to phosphorylate PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3. In contrast, the single member of the class IB PI3K family (PI3K-γ) is composed of the catalytic subunit p110-γ and the p101 regulatory domain. This complex becomes enabled to synthesize PtdIns(3,4,5)P3 after p101 binds to the Gβγ subunit of heterotrimeric G proteins. However, recent studies suggest that PI3K-β can also be activated by G protein–coupled receptors.60-63 Regardless of the mechanism, activation of all type I PI3Ks lead to the synthesis of PtdIns(3,4,5)P3, which in turn can bind to and activate many downstream effector molecules, including the protein kinase Akt. In addition to class I PI3K isoforms that synthesize PtdIns(3,4,5)P3, there are class II PI3Ks that phosphorylate PtdIns(4)P to synthesize PtdIns(3,4)P2, and there are class III PI3Ks that phosphorylate the D3 position of PtdIns to form PtdIns(3)P. Although the role of class I PI3K in platelet adhesion by activation of αIIbβ3 has been extensively studied,64,65 the function of class II and class III PI3Ks in platelets is not well established.
The role of class I PI3K isoforms during platelet plug formation
Human and mouse platelets contain all isoforms of class I PI3K including α, β, and γ and even small amounts of PI3K-δ.64,66-68 The binding of collagen to the platelet GPVI receptor leads to the phosphorylation of the receptor’s FcRγ chain, which allows it to bind to the SH2 domain of PI3K-α, PI3K-β, and PI3K-δ68,69 (Figure 3B). Conversely, activation of the P2Y12 receptor leads to the release of the Gβγ subunit from heterotrimeric G proteins that binds the p101 subunit of PI3K-γ and activates this lipid kinase complex.70-72 Activation of all type I PI3Ks leads to the synthesis of PtdIns(3,4,5)P3, which in turn activates the protein kinase Akt73,74 in platelets. In addition, PtdIns(3,4,5)P3 also facilitates the activation of PLC-γ.75 Together, these signaling events have been shown to contribute to the activation of the small GTPase Rap1b, which is an activator of integrin αIIbβ376 (Figure 3B).
Initial studies using PI3K inhibitors that block all isoforms of PI3K such as wortmannin and LY294002 suggested that PI3K is crucial for platelet adhesion and aggregation under high shear.77,78 However, the exact contribution of individual isoforms of PI3K during platelet activation remained largely unknown. Over the past few years, studies using isoform-specific targeted mouse models and PI3K inhibitors provided strong evidence that the different isoforms of class I PI3K have distinct cellular and physiological functions in diverse cell types including in platelets. For instance, it is now established that PI3K-α is crucial for cell growth and survival. This has implications in oncogenesis, and targeting of PI3K-α is being investigated as a strategy for chemotherapeutics.79 Conversely, PI3K-γ plays an essential role in innate immunity and inflammatory responses,80,81 whereas PI3K-δ is crucial for adaptive immunity involving B and T cells and mast cells.82,83 In platelets, PI3K-δ knockout mice and kinase-deficient PI3K-δ knockin mice have only a minor defect in the GPVI-mediated activation of integrin αIIbβ3.84 This implies that other PI3K isoforms are the major mediators of collagen-induced platelet adhesion and aggregation. Remarkably, in vivo inhibition of PI3K-β impairs the formation of stable αIIbβ3-mediated platelet adhesion and prevents thrombus formation in mice, even though it does not increase the bleeding time.85 This finding suggests that drugs that inhibit PI3K-β might not cause significant hemorrhage when given to patients for brief periods of time, such as during cardiac catheterization.86 Similarly, mice lacking PI3K-γ have defective platelet aggregation upon stimulation with the platelet agonist ADP. Moreover, these mice are less prone to form thrombi in response to intravenous injections with ADP. It should be noted that these mice do not have spontaneous bleeding, again suggesting that PI3K-γ might be a viable target for inhibiting thrombosis formation without inducing excessive hemorrhage.53,87 Additional studies support that the functional roles of PI3K-β and PI3K-γ in platelets are both required for ADP- or TxA2-induced activation of the Rap1b.76 However, only PI3K-β (and not PI3K-γ) was necessary for collagen-stimulated signaling pathways that lead to Akt phosphorylation, Rap1b activation, and platelet aggregation.76 Together, these studies suggest a story that is similar to what is seen in PIP5KI and PLC isoforms. Even though all PI3K class I isoforms can produce PtdIns(3,4,5)P3, the individual isoforms have nonredundant roles in platelets.
Conclusion
Phosphoinositides are tightly regulated both in time and in space. Their relative amounts change within seconds of agonist stimulation of any cell, including platelets. Specific phosphoinositides regulate platelet integrin activation, actin assembly, and secretion. Some phosphoinositides, such as PtdIns(4,5)P2 and PtdIns(3,4,5)P3, can contribute to platelet activation by directly binding to signaling proteins and thereby regulating the function of these proteins. PtdIns(4,5)P2 can also be modified by PLC or PI3K to form second messengers in platelets. Although this process seems overly complex, our recent understanding of the specific roles of individual phosphoinositides has given some insight into why there are so many of these different phosphoinositides and phospholipid kinases. Future studies of these processes should provide greater insight and understanding of both fundamental phospholipid biochemistry and platelet biology and could lead to important advances in treatment and care in the clinical setting.
Acknowledgment
This work was supported in part by the National Institutes of Health: National Heart, Lung, and Blood Institute.
Authorship
Contribution: S.H.M. and C.S.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles S. Abrams, Department of Medicine, University of Pennsylvania School of Medicine, Biomedical Research Building II/III, #812, 421 Curie Blvd, Philadelphia, PA 19104-6160; e-mail: abrams@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal