Key Points
JAK2V617F amplifies in mouse early hematopoietic cells, giving them a proliferative advantage through high cell cycling and low apoptosis.
IFNα prevented myeloproliferative neoplasm development by specifically inhibiting JAK2V617F cells at an early differentiation stage.
Abstract
The acquired gain-of-function V617F mutation in the Janus Kinase 2 (JAK2V617F) is the main mutation involved in BCR/ABL-negative myeloproliferative neoplasms (MPNs), but its effect on hematopoietic stem cells as a driver of disease emergence has been questioned. Therefore, we reinvestigated the role of endogenous expression of JAK2V617F on early steps of hematopoiesis as well as the effect of interferon-α (IFNα), which may target the JAK2V617F clone in humans by using knock-in mice with conditional expression of JAK2V617F in hematopoietic cells. These mice develop a MPN mimicking polycythemia vera with large amplification of myeloid mature and precursor cells, displaying erythroid endogenous growth and progressing to myelofibrosis. Interestingly, early hematopoietic compartments [Lin-, LSK, and SLAM (LSK/CD48−/CD150+)] increased with the age. Competitive repopulation assays demonstrated disease appearance and progressive overgrowth of myeloid, Lin-, LSK, and SLAM cells, but not lymphocytes, from a low number of engrafted JAK2V617F SLAM cells. Finally, IFNα treatment prevented disease development by specifically inhibiting JAK2V617F cells at an early stage of differentiation and eradicating disease-initiating cells. This study shows that JAK2V617F in mice amplifies not only late but also early hematopoietic cells, giving them a proliferative advantage through high cell cycling and low apoptosis that may sustain MPN emergence but is lost upon IFNα treatment.
Introduction
The acquired gain-of-function V617F mutation in the Janus Kinase 2 (JAK2V617F) is the major mutation involved in BCR/ABL-negative classical myeloproliferative neoplasms (MPNs).1-4 It is present in 95% of polycythemia vera (PV) patients and ∼50% to 60% of patients suffering from essential thrombocythemia (ET) or primary myelofibrosis. Retrovirally induced5-7 and transgenic (TG) animals8 have demonstrated that the sole JAK2V617F mutation is able to generate these diseases and that the level of expression of JAK2V617F is crucial in determining MPN diversity.8 Several knock-in (KI) mouse models using the murine gene have been described and all demonstrate that endogenous heterozygous expression of mJAK2V617F leads to a PV-like disease, usually followed by secondary myelofibrosis,9-11 whereas homozygosity increases the severity of the PV-like phenotype.9 In contrast, a mild ET-like disease was reported in a KI model using hJAK2V617F.12
Besides JAK2V617F, several other acquired mutations have been identified in BCR/ABL-negative MPN, although with a frequency often <10%. These mutations target several pathways/complexes: JAK/STAT signaling directly responsible for MPN phenotype, DNA methylation/demethylation, and PRC2 and RNA splicing.13 Functional analyses of several of these additional mutations have questioned the precise contribution of JAK2V617F to MPN disease initiation, as deletion of Tet214,15 or Dnmt3a16 in mice cause hematopoietic stem cell (HSC) amplification and increased self-renewal. On the contrary, the contribution of JAK2V617F to HSC selective amplification has become a matter of controversy.17 Accordingly, analysis of human PV patients has suggested that JAK2V617F amplified terminal stages of hematopoietic cell differentiation without amplifying the early stages, as opposed to Tet2 mutations.18,19 Therefore, the ability of JAK2V617F-positive HSCs to compete with normal HSCs is largely questioned, and actual data suggest that other mutations, occurring before or after JAK2V617F, are instead responsible for clonal emergence of JAK2V617F-positive MPN.
To reevaluate the role of JAK2V617F in HSC amplification, we developed a new conditional JAK2V617F KI model and analyzed early stages of differentiation. We found that endogenous expression of JAK2V617F greatly amplified multipotent progenitor cells and gave a proliferative advantage in a competitive setting. We also showed that interferon-α (IFNα), a drug promoting cycling of dormant cells20,21 and clinically effective in PV patients,22 prevented disease and JAK2V617F-positive disease-initiating cell development. Overall, these results show that JAK2V617F provides a strong competitive advantage to HSCs that may account by itself for MPN emergence and that is lost upon IFNα treatment.
Methods
Generation of the conditional JAK2V617F KI mice
Animal experiments were approved by the Institut Gustave Roussy review board, protocol no. 2012-061. The method to generate and genotype the conditional flexed JAK2 (JAK2FLEX/+ or L2/+) KI mice, backcrossed >15 times with C57Bl/6 mice, is described in supplemental Data and Figure 1 (available on the Blood Web site). To express the mutation, KI mice were crossed with CmvCre TG mice to validate the KI construct and with VavCre TG mice23,24 for the rest of the study. We termed these heterozygous recombined JAK2V617F/WT mice (JAK2VF/+), with a wild-type (WT) and a mutated Jak2 allele, as JAK2V617F KI mice.
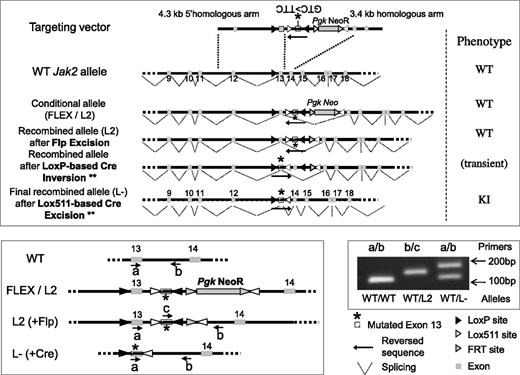
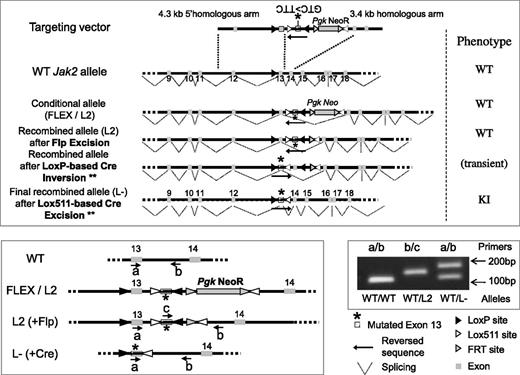
Schematic representation of the targeting vector and resulting modified allele to generate the Jak2V617F KI mouse model. Homologous recombination into the Jak2 WT allele of mouse embryonic stem (ES) cells resulted into the FLEX (F) or L2 conditional allele. A correctly targeted ES clone was injected into blastocyst stage embryos to generate L2 chimeric mice (WT phenotype). Chimeras were bred with flippase (FLP recombinase) TG mice to remove the FRT-flanked selection PgkNeoR cassette from the progeny. To generate the L- KI offspring, expressing the mutated exon 13 and the resulting JAK2V617F protein, KIflex(F)/+ animals were crossed with TG animals expressing the Cre recombinase. Cre recombination induced the LoxP site-directed inversion of a KI construct, resulting in the Lox511-directed excision of the WT exon13 and transcription of the mutated exon13 into the G1849U-mutated mRNA that results into the JAK2V617F protein translation (KI phenotype). The WT, L2 (FLEX), and L- alleles could be discriminated using PCR analysis with different primers (a, b, c) whose location is represented in the figure. A typical result from tail-DNA genotyping, showing correct heterozygous recombination (L- construct), is shown with the a (TGTCTTACTAAAGCCCAGGTGATGG)/b (GCTCCAGGGTTACACGAGTC) couple of primers resulted into the 105-bp, 186-bp, and 884-bp (the latter not visible with our PCR conditions) PCR-amplified fragments within the WT, L-, and L2 allele, respectively. The c (GTCTGTCCAAAGAGTCTGTAAGTAC)/b couple of primers amplified a fragment (144 bp) only within the L2 allele. ** indicates the Lox511-based inversion can also precede the LoxP-based excision, resulting in an identical KI phenotype.
Schematic representation of the targeting vector and resulting modified allele to generate the Jak2V617F KI mouse model. Homologous recombination into the Jak2 WT allele of mouse embryonic stem (ES) cells resulted into the FLEX (F) or L2 conditional allele. A correctly targeted ES clone was injected into blastocyst stage embryos to generate L2 chimeric mice (WT phenotype). Chimeras were bred with flippase (FLP recombinase) TG mice to remove the FRT-flanked selection PgkNeoR cassette from the progeny. To generate the L- KI offspring, expressing the mutated exon 13 and the resulting JAK2V617F protein, KIflex(F)/+ animals were crossed with TG animals expressing the Cre recombinase. Cre recombination induced the LoxP site-directed inversion of a KI construct, resulting in the Lox511-directed excision of the WT exon13 and transcription of the mutated exon13 into the G1849U-mutated mRNA that results into the JAK2V617F protein translation (KI phenotype). The WT, L2 (FLEX), and L- alleles could be discriminated using PCR analysis with different primers (a, b, c) whose location is represented in the figure. A typical result from tail-DNA genotyping, showing correct heterozygous recombination (L- construct), is shown with the a (TGTCTTACTAAAGCCCAGGTGATGG)/b (GCTCCAGGGTTACACGAGTC) couple of primers resulted into the 105-bp, 186-bp, and 884-bp (the latter not visible with our PCR conditions) PCR-amplified fragments within the WT, L-, and L2 allele, respectively. The c (GTCTGTCCAAAGAGTCTGTAAGTAC)/b couple of primers amplified a fragment (144 bp) only within the L2 allele. ** indicates the Lox511-based inversion can also precede the LoxP-based excision, resulting in an identical KI phenotype.
Treatment and analysis of mice
For bone marrow (BM) transplantation, 3 × 106 C57/Bl6/6J BM cells were engrafted into lethally irradiated (9.5 Gy) congenic recipient mice. For competitive BM transplantation, lethally irradiated Ly5.1 WT recipient mice were injected with BM cells from Ly5.1+2 WT donor mice mixed with BM cells from Ly5.2 VavCre/JAK2VF/+ mice. Murine IFNα (Miltenyi Biotech; 107 UI/mL) was subcutaneously injected every day at a dose of 3 × 104 IU in 0.2 mL phosphate-buffered saline/mouse (1.5 × 106 IU/kg). Control vehicle mice were injected with phosphate-buffered saline.
Hemoglobin, mean corpuscular volume, hematocrit, red blood cell (RBC), platelet, and white blood cell (WBC) counts were determined using an automated counter (MS9; Schloessing Melet, France) on blood collected from the retro-orbital plexus in citrated tubes. BM cells were removed by flushing both femurs. Spleens were weighed and single cell suspensions were prepared. For histopathology analysis, spleens were fixed in formaldehyde and sections (4.5 µm) were stained with hematoxylin/eosin, periodic acid Schiff, and Giemsa for cytology analysis. Reticulin fibers were revealed by silver staining according to the Gordon Sweet method. Flow cytometry of blood, BM, and spleen was used as described in supplemental Data.
Progenitor cell study
Progenitor cell assays were carried out in 1 mL methylcellulose “MethoCult 32/34” (Stemcell Technologies) without stimulus or maximally stimulated by 10 ng/mL IL3, IL6, TPO, 100 ng/mL SCF, and 2 IU/mL EPO. Cultures in duplicate or quadruplicate were scored after 2 days for colony forming unit-erythroid (CFU-E) assays and 8 days for burst forming unit-erythroid (BFU-E) and colony forming unit granulocyte-macrophage (CFU-GM) assays. Total progenitor cell number was calculated assuming that one femur represents 6% of the total marrow and from the total number of cells isolated from the spleen.
Cell cycle and apoptosis analysis
BM or spleen cells were stained for LSK and SLAM isolation. For apoptosis studies, cells were stained with Annexin V-PB antibody (Biolegend). For cell cycle status, cells were fixed, permeabilized, and then labeled with Ki-67-FITC (Becton Dickinson) and Hoechst 33342 (Invitrogen). Flow cytometry was performed using an LSR II analyzer (Becton Dickinson).
Statistical analysis
Results are presented as mean ± SEM and data were analyzed with the 2-tailed Student t test.
Results
Constitutive expression of JAK2V617F results in PV-like phenotype
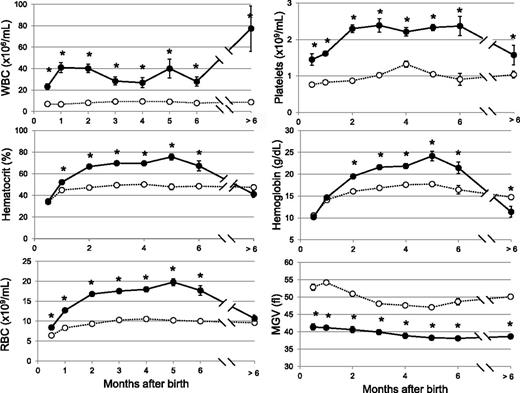
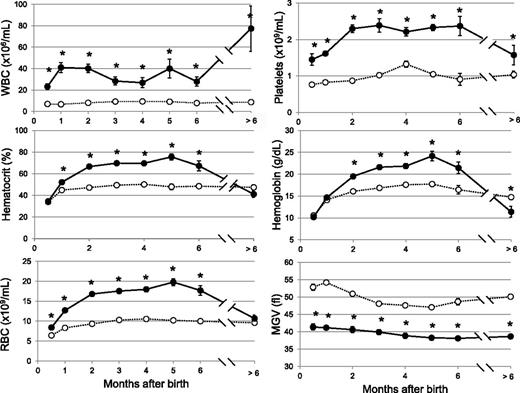
We generated conditional JAK2V617F KI mice using the FLEX switch strategy25 (Figure 1; supplemental Data). We crossed the JAK2FLEX/+ KI mice with TG mice expressing the Cre recombinase under the control of the Vav promoter.26,27 VavCre TG mice targets in vivo Cre recombination in HSCs27 and some endothelial cells.24 Allele specific reverse-transcription polymerase chain reaction in BM revealed an equal amount of JAK2WT and JAK2V617F mRNA, demonstrating that all BM cells were recombined with a normal transcriptional activity of the mutated locus (supplemental Figure 1). These conditional VavCre/JAK2VF/+ animals were born with severe thrombocytosis and leukocytosis (Figure 2). Surprisingly, the hemoglobin and hematocrit levels remained normal during the first month of life. RBC number was slightly in excess in 2-week-old mice, but severe microcytosis precluded any effect on hematocrit (Figure 2). At 2 to 3 months of age, hematocrit values were elevated and reached a plateau for 5 months. Reticulocyte counts were elevated (17 ± 2%, controls 2.8 ± 0.4%, n = 13). Platelet numbers rapidly increased and reached a plateau for 6 months. In aged mice, WBCs reached high values, but platelet counts along with hemoglobin levels decreased to subnormal levels, as observed during myelofibrosis development in other models (Figure 2).5,28
Blood cell parameters in JAK2V617F KI mice. Blood parameters from KI (filled circles, n = 5-41) and WT littermate (open circles, n = 5-24) mice were studied from 2 weeks of age to over 6 months of age. Values were grouped to the closest month. Hematocrit values at 2-3 months were 70 ± 2% n = 13, controls 49 ± 1% n = 13. Platelet numbers reach at 2 months 2.3 ± 0.1 × 109/mL, n = 31, controls 0.84 ± 0.04 × 109/mL, n = 20. WBCs remained high for 6 months (36 ± 2 × 106/mL, n = 117, controls 8.0 ± 0.3 × 106/mL, n = 89). After 6 months, WBCs reached 77 ± 21 × 106/mL n = 5; controls: 8.7 ± 0.7 × 106/mL, n = 8; but platelet decreased to subnormal levels (1.6 ± 0.21 × 109/mL, n = 5; controls 1.03 ± 0.09 × 109/mL, n = 8) along with hemoglobin levels (11.4 ± 1.2 g/L, n = 5, control values 14.7 ± 0.5 g/L, n = 8). Results are mean value ± SEM, *P ≤ .05 with the 2-tailed unpaired Student t test. MCV, mean corpuscular volume; MGV, mean globular volume; MPV, mean platelet volume.
Blood cell parameters in JAK2V617F KI mice. Blood parameters from KI (filled circles, n = 5-41) and WT littermate (open circles, n = 5-24) mice were studied from 2 weeks of age to over 6 months of age. Values were grouped to the closest month. Hematocrit values at 2-3 months were 70 ± 2% n = 13, controls 49 ± 1% n = 13. Platelet numbers reach at 2 months 2.3 ± 0.1 × 109/mL, n = 31, controls 0.84 ± 0.04 × 109/mL, n = 20. WBCs remained high for 6 months (36 ± 2 × 106/mL, n = 117, controls 8.0 ± 0.3 × 106/mL, n = 89). After 6 months, WBCs reached 77 ± 21 × 106/mL n = 5; controls: 8.7 ± 0.7 × 106/mL, n = 8; but platelet decreased to subnormal levels (1.6 ± 0.21 × 109/mL, n = 5; controls 1.03 ± 0.09 × 109/mL, n = 8) along with hemoglobin levels (11.4 ± 1.2 g/L, n = 5, control values 14.7 ± 0.5 g/L, n = 8). Results are mean value ± SEM, *P ≤ .05 with the 2-tailed unpaired Student t test. MCV, mean corpuscular volume; MGV, mean globular volume; MPV, mean platelet volume.
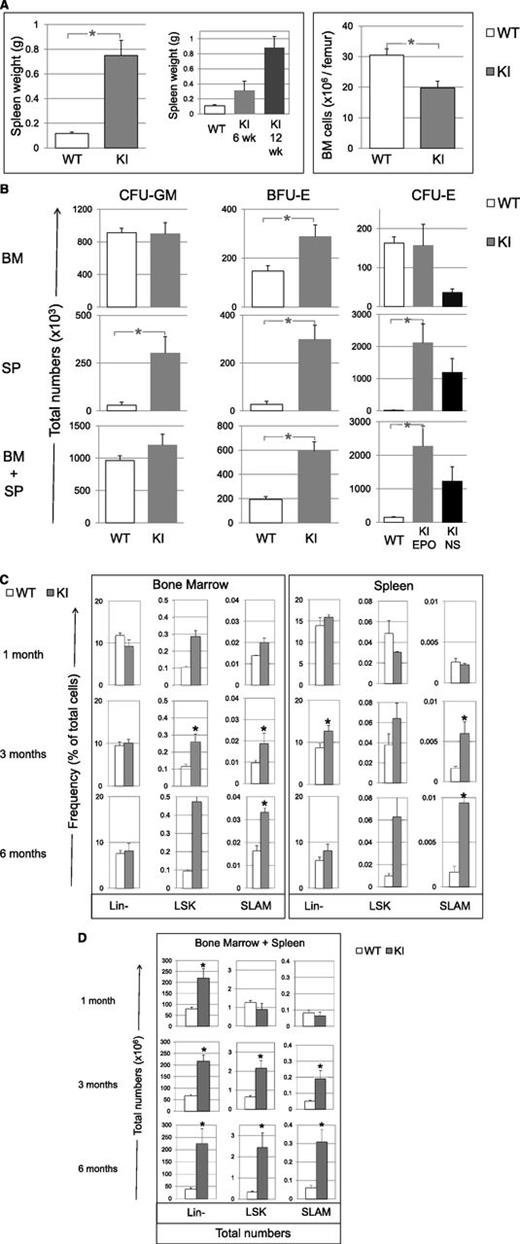
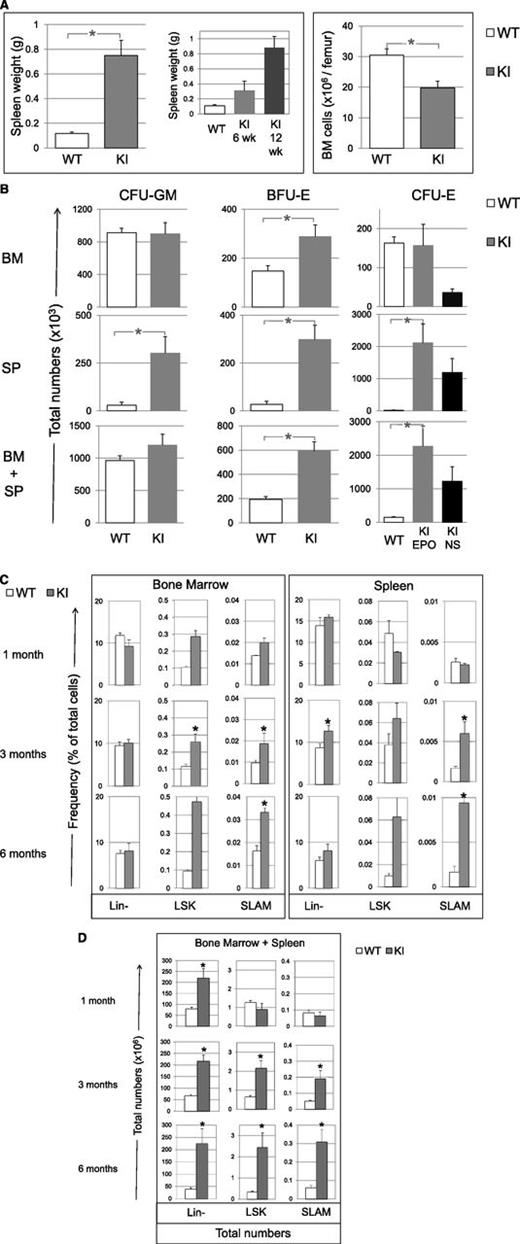
Hematopoietic tissues were abnormal. BM cellularity was reduced (60% of control) (Figure 3A) with an increase in the percentage of granulocytes (from 41 ± 2% in WT littermates to 70 ± 3%; P = .02) and a decrease in both mature erythroblasts (from 30 ± 4% in WT to 8 ± 1%; P = .03), as previously reported in JAK2V617F mouse models,5,10 and B cells (from 20 ± 2% in WT to 10 ± 3%). Splenomegaly was mild at 6 weeks (3-fold) but severe at 12 weeks (8-fold) (Figure 3A) and was due to an increase in erythroblasts (from 7 ± 5% in WT to 14 ± 2%) and myeloid cells (from 2 ± 1% in WT to 18 ± 4%), with a decreased percentage of B cells (from 51 ± 3% in WT to 27 ± 4%). In aged mice (>6 months), we observed high-grade fibrosis in BM and spleen and extra-medullary hematopoiesis in liver (supplemental Figure 2).
Tissue cellularity, progenitor and early cell content in KI (gray bars), and WT littermate (white bars) mice. (A) Spleen weight (also showing spleens collected in 6- and 12-week-old KI mice) and BM cell content (6-12 weeks) per femur in KI (n = 11) and WT littermate (n = 9) mice. (B) Total numbers (×103) in BM (top), spleen (SP, middle) and cumulative numbers (×103) in BM plus spleen (BM+SP) of CFU-GM, BFU-E and CFU-E from KI (n = 9) and WT littermate (n = 7) mice. CFU-E numbers from KI mice growing without added Epo (endogenous colonies) are represented in the black bars (NS for nonstimulated). No endogenous CFU-E was found in organs from WT littermate mice. The calculation is detailed in “Methods.” (C) Percentages of Lin-, LSK, and SLAM cells in BM and spleen from KI and WT littermates measured by FACS at 1, 3, and 6 months of age, n = 3 mice for 1- and 6-month-old mice and n = 8-12 mice for 3-month-old mice. (D) Total numbers (×106) of Lin-, LSK, and SLAM (LSK, CD48−, CD150+) cells in BM plus spleen from KI and WT littermates measured at 1, 3, and 6 months of age. Calculation is detailed in “Methods” from percentage of Lin-, LSK, and SLAM cells represented in C. n = 3 for SLAM, LSK, and Lin- in 6-month-old mice or 8-9 mice for 3-month-old mice. Results are mean value ± SEM. *P ≤ .05 with the 2-tailed unpaired Student t test.
Tissue cellularity, progenitor and early cell content in KI (gray bars), and WT littermate (white bars) mice. (A) Spleen weight (also showing spleens collected in 6- and 12-week-old KI mice) and BM cell content (6-12 weeks) per femur in KI (n = 11) and WT littermate (n = 9) mice. (B) Total numbers (×103) in BM (top), spleen (SP, middle) and cumulative numbers (×103) in BM plus spleen (BM+SP) of CFU-GM, BFU-E and CFU-E from KI (n = 9) and WT littermate (n = 7) mice. CFU-E numbers from KI mice growing without added Epo (endogenous colonies) are represented in the black bars (NS for nonstimulated). No endogenous CFU-E was found in organs from WT littermate mice. The calculation is detailed in “Methods.” (C) Percentages of Lin-, LSK, and SLAM cells in BM and spleen from KI and WT littermates measured by FACS at 1, 3, and 6 months of age, n = 3 mice for 1- and 6-month-old mice and n = 8-12 mice for 3-month-old mice. (D) Total numbers (×106) of Lin-, LSK, and SLAM (LSK, CD48−, CD150+) cells in BM plus spleen from KI and WT littermates measured at 1, 3, and 6 months of age. Calculation is detailed in “Methods” from percentage of Lin-, LSK, and SLAM cells represented in C. n = 3 for SLAM, LSK, and Lin- in 6-month-old mice or 8-9 mice for 3-month-old mice. Results are mean value ± SEM. *P ≤ .05 with the 2-tailed unpaired Student t test.
These data show that heterozygous endogenous JAK2V617F expression in hematopoietic cells leads to hyperplasia of mature and maturing erythroid, granulocytic, and megakaryocytic cells in blood and hematopoietic tissues that models human PV with secondary myelofibrosis.
Endogenous JAK2V617F expression increases early stages of differentiation
We next studied the effect of JAK2V617F on early stages of differentiation. We first analyzed progenitor cell numbers from VavCre/JAK2VF/+ animals using clonogenic assays. In the marrow, the frequencies of all progenitor cells except CFU-E increased (supplemental Figure 3). Taking into account the decrease in BM cellularity (Figure 3A), the total numbers of myeloid progenitors (CFU-GM) and late erythroid progenitors (CFU-E) did not differ from littermates, but early erythroid progenitor cells (BFU-E) were 2-fold increased (Figure 3B). In the spleen, frequencies of all progenitor cells were increased (supplemental Figure 3) and taking into account splenomegaly, the myeloid progenitor cell compartment was largely amplified, particularly CFU-E (124-fold) (Figure 3B), with 56% of them being Epo-independent. Finally, cumulative numbers of CFU-GM, BFU-E, and CFU-E in marrow plus spleen were increased by 1.2-, 3-, and 15-fold, respectively (Figure 3B).
We then analyzed by flow cytometry Lin-, LSK, and LSK/CD48-/CD150+ cells, so-called SLAM cells, which are highly enriched (1 of 2.2)29 in long-term reconstituting activity (LT-HSC) (supplemental Figure 4). In 3-month-old mice, JAK2V617F KI animals demonstrated in marrow no change in the frequency of Lin- cells but an increase in LSK (×2.3) and SLAM (×2) cells (Figure 3C). In spleen, the percentages of Lin-, LSK, and SLAM cells increased 1.5-, 1.7-, and 3.7-fold, respectively. We noticed a progressive increase with age. A normal frequency was observed in 1-month-old mice, but the percentages of BM and spleen LSK and SLAM cells were increased in 6-month-old mice more than in 3-month-old mice. Overall, we observed a 3-, 4-, and 5-fold increase in the total numbers (BM plus spleen) of Lin- cells in 1-, 3-, and 6-month-old mice, respectively. In contrast, there was no increase in the total numbers of LSK and SLAM cells in 1-month-old mice but a 3- or 7-fold and a 4- or 6-fold increase in the total numbers of LSK and SLAM cells in 3- or 6-month-old mice, respectively (Figure 3D). One possible pitfall could be the upregulation of Sca-I by JAK2V617F, but SLAM cells, analyzed regardless of Sca-I, were similarly increased. Related to c-Kit+/Lin- progenitor cells (LK), the total numbers of MEP, GMP, and CMP augmented in 3- and 6-month-old mice, mainly due to the spleen content with marked and early increase in MEP (supplemental Figures 4 and 5). Overall, the differences between KI and WT mice in the pools of LSK, GMP, CMP, and particularly SLAM cells increased with the age, whereas these differences in MEP and Lin- pools seemed precociously established. These results show that endogenous JAK2V617F progressively amplifies the earliest stages of differentiation with the age while late stages are already amplified in young mice.
JAK2V617F provides a competitive advantage to hematopoietic cells at early stages of differentiation
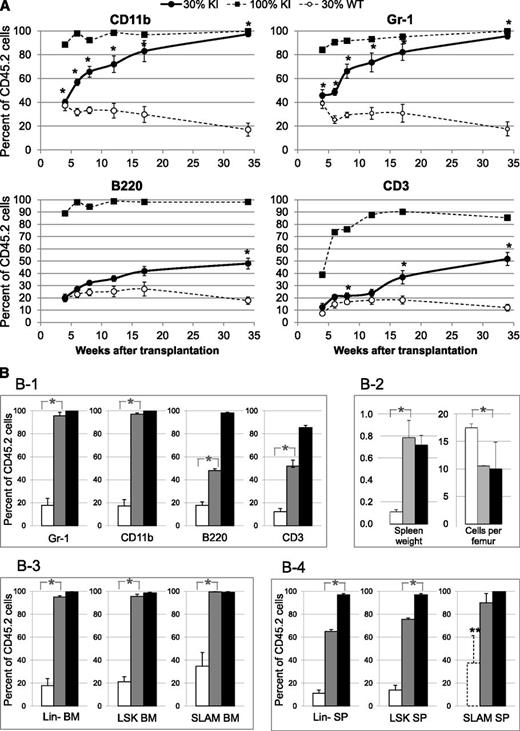
We next investigated if JAK2V617F expression confers a proliferative advantage to LT-HSC using competitive grafts in CD45.1 WT lethally irradiated recipient animals transplanted with 30% CD45.2 KI and 70% CD45.1+2 WT BM cells collected from 3-month-old donor mice. As a WT control, we used 30% CD45.2 WT and 70% CD45.1+2 WT grafts and as a control for full disease engraftment, we used 100% CD45.2 KI grafts.
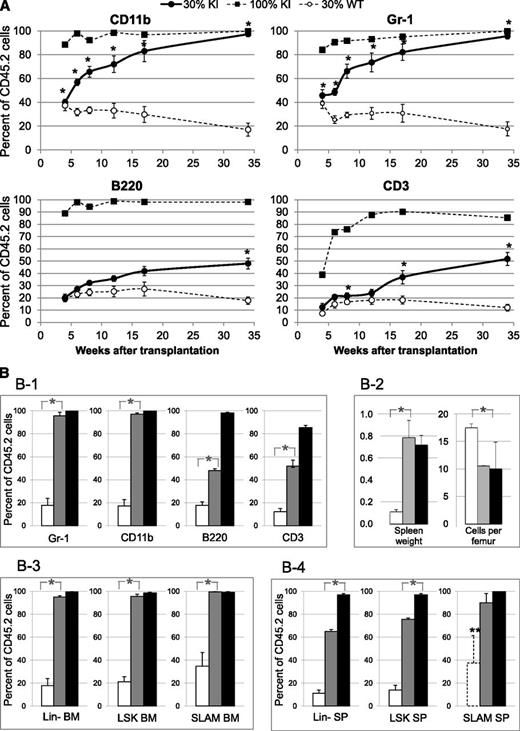
The disease was transmitted to all recipients grafted with 30% KI graft (supplemental Figure 6). Blood KI myeloid cells (CD45.2/CD11b/Gr-1) increased from ∼40% to ∼90% (similarly to mice transplanted with 100% KI cells) from 4 to 34 weeks posttransplantation (Figure 4A). In contrast, the percentages of blood KI lymphoid cells (CD45.2/B220/CD3) only reached ∼50% at 34 weeks posttransplant (Figure 4A-B). Mice were killed 34 weeks posttransplant to analyze chimerism in Lin-, LSK, and SLAM cells. Strikingly, the percentages of BM Lin-, LSK, and SLAM cells from KI origin in mice grafted with 30% KI cells reached similarly high percentages (95 ± 1%, 96 ± 2%, and 99 ± 1%, respectively) as those (99 ± 1%, 98 ± 2%, and 99 ± 1%, respectively) found in mice transplanted with 100% KI BM cells (Figure 4B). The percentages of spleen KI Lin-, LSK, and SLAM cells (65 ± 2%, 76 ± 1%, and 90 ± 8%, respectively) were slightly lower (Figure 4B). The extent of splenomegaly and the reduction in marrow cellularity were similar in mice transplanted with 30% and 100% KI cells, showing that disease severity was comparable in both groups (Figure 4B).
Competitive grafts in recipient mice transplanted with 3-month-old donor mice showing proliferative advantage of KI cells over WT cells. (A) Percentage of CD45.2+ blood myeloid (CD11b+ or Gr-1+) and lymphoid (B220+ or CD3+) cells from KI or WT origin in recipient mice showing progressive overgrowth of KI cells in competitive grafts. CD45.1 recipient mice were transplanted with either 100% KI cells (CD45.2) (■, dashed line, n = 5) or a mixture of 30% KI cells (CD45.2) plus 70% WT CD45.1+2 cells (●, solid line, n = 5). As a control, CD45.1 recipient mice were transplanted with a mixture of 30% WT CD45.2 cells and 70% WT CD45.1+2 cells (○, dashed line, n = 5). (B) Percentages of CD45.2+ cells from KI or WT origin in blood and tissues analyzed 34 weeks after competitive grafts showing progressive overgrowth of KI cells in late and early stages of differentiation. CD45.1 recipient mice were transplanted with either 100% KI cells (CD45.2) (black columns) or a mixture of 30% KI cells (CD45.2) plus 70% CD45.1+2 WT cells (gray columns). As a control, CD45.1 recipient mice were transplanted with a mixture of 30% WT CD45.2 cells plus 70% WT CD45.1+2 cells (white columns). (B-1) CD45.2, Gr-1, CD11b, B220, and CD3-positive cells were determined in blood. (B-2) Spleen weight (g) and marrow cellularity (×106 cells/femur) in recipient mice. (B-3-4) CD45.2+, Lin-, LSK, and SLAM (LSK, CD48−, CD150+) cells were determined in BM (3) or spleen (4). ** indicates that the numbers of SLAM cells detected in the WT spleen samples were too low (10 ± 5 cells) to provide a reliable value. Data are mean values ± SEM, n = 3. *P ≤ .05 with the 2-tailed unpaired Student t test.
Competitive grafts in recipient mice transplanted with 3-month-old donor mice showing proliferative advantage of KI cells over WT cells. (A) Percentage of CD45.2+ blood myeloid (CD11b+ or Gr-1+) and lymphoid (B220+ or CD3+) cells from KI or WT origin in recipient mice showing progressive overgrowth of KI cells in competitive grafts. CD45.1 recipient mice were transplanted with either 100% KI cells (CD45.2) (■, dashed line, n = 5) or a mixture of 30% KI cells (CD45.2) plus 70% WT CD45.1+2 cells (●, solid line, n = 5). As a control, CD45.1 recipient mice were transplanted with a mixture of 30% WT CD45.2 cells and 70% WT CD45.1+2 cells (○, dashed line, n = 5). (B) Percentages of CD45.2+ cells from KI or WT origin in blood and tissues analyzed 34 weeks after competitive grafts showing progressive overgrowth of KI cells in late and early stages of differentiation. CD45.1 recipient mice were transplanted with either 100% KI cells (CD45.2) (black columns) or a mixture of 30% KI cells (CD45.2) plus 70% CD45.1+2 WT cells (gray columns). As a control, CD45.1 recipient mice were transplanted with a mixture of 30% WT CD45.2 cells plus 70% WT CD45.1+2 cells (white columns). (B-1) CD45.2, Gr-1, CD11b, B220, and CD3-positive cells were determined in blood. (B-2) Spleen weight (g) and marrow cellularity (×106 cells/femur) in recipient mice. (B-3-4) CD45.2+, Lin-, LSK, and SLAM (LSK, CD48−, CD150+) cells were determined in BM (3) or spleen (4). ** indicates that the numbers of SLAM cells detected in the WT spleen samples were too low (10 ± 5 cells) to provide a reliable value. Data are mean values ± SEM, n = 3. *P ≤ .05 with the 2-tailed unpaired Student t test.
These results show that endogenous JAK2V617F expression confers a selective advantage to LT-HSC and myeloid cell progenies.
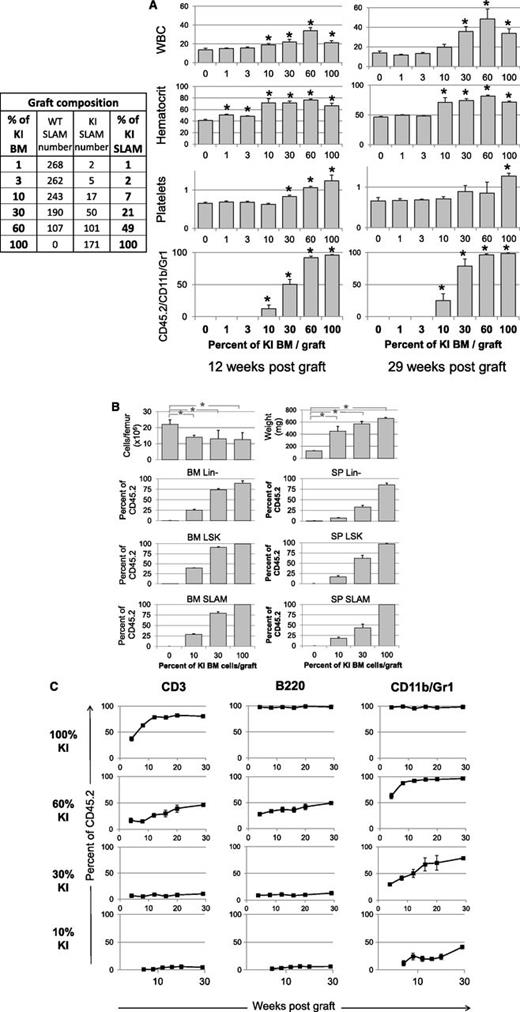
Our 30% KI graft initiated from 3-month-old mice should correspond to a 46% KI graft at the stem cell level due to the previously shown 2-fold BM SLAM amplification. Therefore, to know the minimum graft necessary for disease emergence, we developed competitive grafts from young donor mice, including different KI/WT BM cell percentages corresponding to similar KI/WT SLAM cell percentages and known KI SLAM numbers (Figure 5A). Results showed that 30% KI BM containing ≥50 KI SLAM cells always generated a long-term disease (29 weeks posttransplant) with polycythemia, leukocytosis, thrombocytosis, splenomegaly, and low BM cellularity, although long-term thrombocytosis was not always observed (Figure 5A-B). In contrast, only 3 of 5 recipients grafted with 10% KI BM or 17 KI SLAM cells developed the disease. Experiments including 10 and 15 mice confirmed that 10% KI BM grafts generated only long-term disease (15 months) in ∼50% recipients (5/10 and 7/15). These results suggest that in competition assays, ∼30 JAK2V617F SLAM cells are sufficient to generate MPN with complete penetrance. Interestingly, the percentages of blood lymphocytes from KI origin 30 weeks posttransplant were similar or lower to the percentages of KI SLAM cells initially transplanted, suggesting no or very delayed KI lymphoid cell amplification (Figure 5C). In contrast, as previously shown, the percentages of KI blood myeloid cells, BM/spleen Lin-, LSK, and SLAM cells were higher than the initial KI SLAM cell input, showing overgrowth of these cells in recipients (Figure 5B-C).
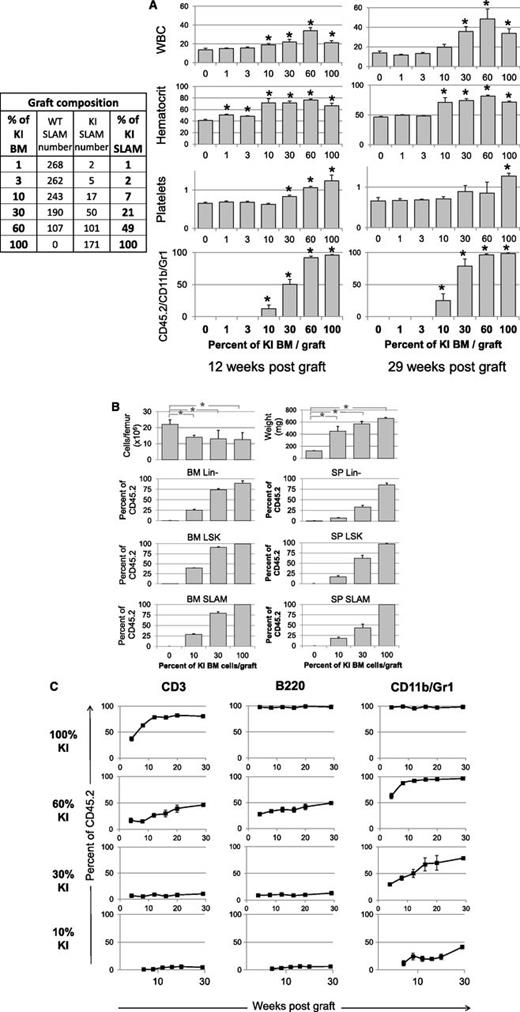
Competitive grafts with different ratio of KI/WT BM cells showing disease propagation from a low number of KI SLAM cells. (A) Left: composition in WT/KI SLAM (LSK, CD48−, CD150+) cells, determined by FACS analysis, in the 3 × 106 cells grafts mixing different percentages of KI and WT BM cells from 1-month-old donor mice. Right: WBC (106/mL), hematocrit (%), and platelet (109/mL) levels and frequency of blood KI myeloid cells (CD45.2/CD11b/Gr-1) measured 12 and 29 weeks after transplantation of BM samples containing 0%, 1%, 3%, 10%, 30%, 60%, and 100% KI BM cells (n = 5/graft mixture). Results show that a disease may develop (3/5 recipients) from a graft containing 10% KI BM cells or 17 KI SLAM cells. (B) BM cellularity, spleen weight, and Lin-, LSK, and SLAM cells from KI origin found 29 weeks after transplantation in mice transplanted with 0%, 10%, 30%, and 100% KI BM cells. Results show low BM cellularity, splenomegaly, and amplification of early cells from the initial 7% and 21% KI SLAM cell input present in the 10% and 30% KI BM graft. (C) Percent of CD3, B220, and CD11b/Gr-1 cells from KI origin in the blood of mice transplanted with 10%, 30%, 60%, and 100% KI BM cells. Results show that myeloid cells but not lymphoid cells were amplified after transplantation from the initial 7%, 21%, and 49% KI SLAM cell input present in the 10%, 30%, and 60% KI BM graft. Data are mean values ± SEM, n = 5/graft mixture, except for mice grafted with 10% KI cells in graphs B and C, where only 3 mice developed the disease, the 2 other mice having no KI cells detectable 6 months after transplantation. *P ≤ .05 with the 2-tailed unpaired Student t test compared with recipients grafted with 0% KI cells.
Competitive grafts with different ratio of KI/WT BM cells showing disease propagation from a low number of KI SLAM cells. (A) Left: composition in WT/KI SLAM (LSK, CD48−, CD150+) cells, determined by FACS analysis, in the 3 × 106 cells grafts mixing different percentages of KI and WT BM cells from 1-month-old donor mice. Right: WBC (106/mL), hematocrit (%), and platelet (109/mL) levels and frequency of blood KI myeloid cells (CD45.2/CD11b/Gr-1) measured 12 and 29 weeks after transplantation of BM samples containing 0%, 1%, 3%, 10%, 30%, 60%, and 100% KI BM cells (n = 5/graft mixture). Results show that a disease may develop (3/5 recipients) from a graft containing 10% KI BM cells or 17 KI SLAM cells. (B) BM cellularity, spleen weight, and Lin-, LSK, and SLAM cells from KI origin found 29 weeks after transplantation in mice transplanted with 0%, 10%, 30%, and 100% KI BM cells. Results show low BM cellularity, splenomegaly, and amplification of early cells from the initial 7% and 21% KI SLAM cell input present in the 10% and 30% KI BM graft. (C) Percent of CD3, B220, and CD11b/Gr-1 cells from KI origin in the blood of mice transplanted with 10%, 30%, 60%, and 100% KI BM cells. Results show that myeloid cells but not lymphoid cells were amplified after transplantation from the initial 7%, 21%, and 49% KI SLAM cell input present in the 10%, 30%, and 60% KI BM graft. Data are mean values ± SEM, n = 5/graft mixture, except for mice grafted with 10% KI cells in graphs B and C, where only 3 mice developed the disease, the 2 other mice having no KI cells detectable 6 months after transplantation. *P ≤ .05 with the 2-tailed unpaired Student t test compared with recipients grafted with 0% KI cells.
These results show that endogenous JAK2V617F expression generates MPN in mice from a low number of HSCs and confers no selective advantage or bias to lymphoid cells.
Effect of long-term treatment by IFNα
Previous results in KI mice have shown that a JAK2 inhibitor could not eradicate disease-initiating cells.11 Therefore, we investigated if another drug, IFNα, could hamper this early cell reservoir for MPN propagation. IFNα seemed a good candidate, because it induces HSC exhaustion20,21 and molecular response in patients suffering from PV through still unclear mechanisms.22,30
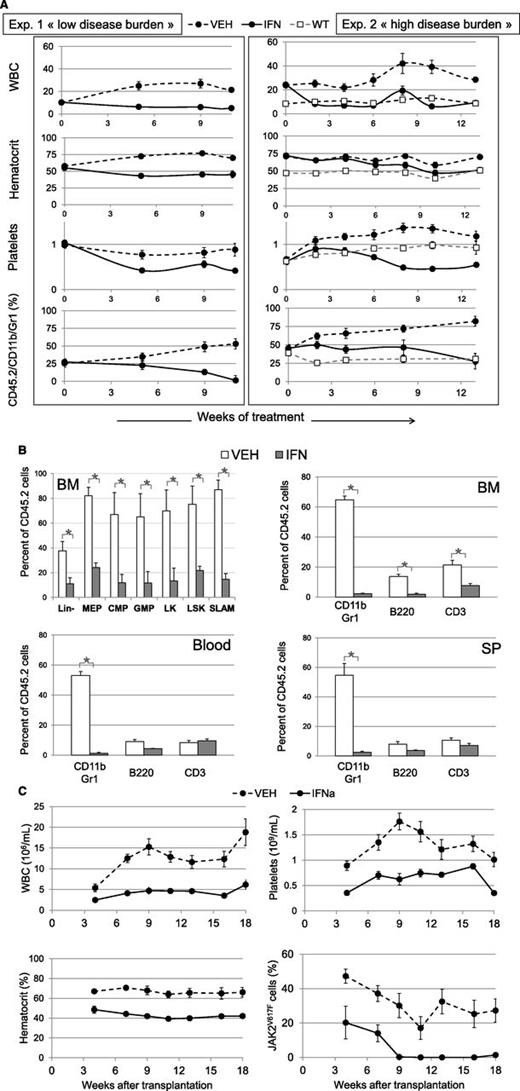
IFNα treatment was initiated in 2 groups of CD45.1 WT recipient mice 7 weeks after the transplantation of 30% KI BM cells collected from 1- or 3-month-old donor mice in order to generate low or high disease burden, as previously shown. In both groups of mice, IFNα treatment induced a rapid suppression of leukocytosis without leukopenia (Figure 6A). All cell types dropped, but mainly B cells and the percentage of T cells, particularly CD3+/LY6C+, and myeloid cells increased (supplemental Figure 7). Platelet levels also dropped quickly after IFNα treatment but reached a plateau lower than control (Figure 6A). Hematocrit, hemoglobin, and RBC counts normalized early or late after treatment according to low or high disease severity (Figure 6A). Microcytosis was corrected after polycythemia (supplemental Figure 8), suggesting correction of iron deficiency.10 The percentage of myeloid cells from KI origin (CD45.2/CD11b/Gr-1) in vehicle-treated mice increased with time, highlighting disease progression. IFNα cancelled out this progression, and myeloid chimerism at the end of treatment was reduced to ∼0% or 27 ± 10% vs 53 ± 3% or 85 ± 6% in vehicle-treated mice in the first or second group of mice, respectively (Figure 6A). Chimerism was also strongly reduced in BM lymphoid, myeloid progenitor, and SLAM cells but not in blood and spleen lymphocytes (Figure 6B; supplemental Figure 9). Splenomegaly was slightly reduced in the severe disease group (323 ± 4 mg vs 485 ± 20 mg). In the low severity group, splenomegaly was strongly reduced (176 ± 3 mg vs 580 ± 27 mg; P = .001) with normalization of its architecture. BM cellularity was increased. Myeloid and MKC hyperplasia were suppressed in BM and spleen (supplemental Figure 10).
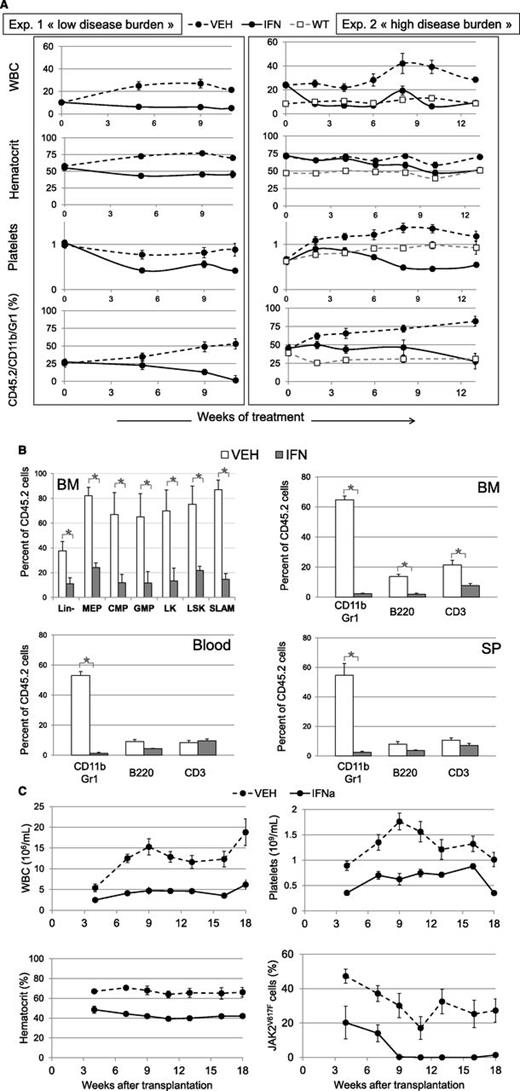
Effect of IFNα treatment on hematological parameters and malignant JAK2V617F-positive cell proliferation. KI recipient animals (●) were transplanted with 30% CD45.2 KI and 70% CD45.1+2 WT BM cells from 1 (exp. 1, left) or 3 (exp. 2, right) month-old donor mice and treated for 11 (exp. 1) or 13 (exp. 2) weeks with IFNα (continuous line) or vehicle (dashed line) starting 7 weeks after transplantation. Control WT recipient animals (□) represent untreated mice grafted with 30% CD45.2 WT and 70% CD45.1+2 WT cells. (A) Variations in WBC (106/mL), hematocrit (%), platelet (109/mL), and the percentage of KI-derived myeloid blood cells (Gr-1/CD11b/CD45.2) analyzed during 11 or 13 weeks of treatment of primary recipient animals. Data are mean values ± SEM of 10 or 5 IFNα-treated, 10 or 5 vehicle-treated (exp.1 or 2, respectively) and 5 nontreated (WT) mice. (B) Variations after 11 weeks of treatment (from exp. 1) in the percentages of cells from KI origin in BM, spleen, and blood. Are represented the early cell pools in BM (Lin-, MEP, CMP, GMP, LK, LSK, and SLAM cells determined as supplemental figure 4) (upper left) and CD11b/Gr1, B220, CD3 in BM (upper right), blood (lower left) and spleen (lower right). Data are mean values ± SEM, n = 3. (C) Secondary transplantation from exp. 2: variations in the hematological parameters and the percentages of KI-derived cells, analyzed by allele-specific PCR, of animals transplanted with BM from donors treated during 13 weeks with IFNα (continuous line) and vehicle (dashed line). Data are mean values ± SEM of 2 groups of 5 mice, each transplanted with BM from 2 different donors. Results from the secondary transplantation of exp. 1 are figured in supplemental Figure 11.
Effect of IFNα treatment on hematological parameters and malignant JAK2V617F-positive cell proliferation. KI recipient animals (●) were transplanted with 30% CD45.2 KI and 70% CD45.1+2 WT BM cells from 1 (exp. 1, left) or 3 (exp. 2, right) month-old donor mice and treated for 11 (exp. 1) or 13 (exp. 2) weeks with IFNα (continuous line) or vehicle (dashed line) starting 7 weeks after transplantation. Control WT recipient animals (□) represent untreated mice grafted with 30% CD45.2 WT and 70% CD45.1+2 WT cells. (A) Variations in WBC (106/mL), hematocrit (%), platelet (109/mL), and the percentage of KI-derived myeloid blood cells (Gr-1/CD11b/CD45.2) analyzed during 11 or 13 weeks of treatment of primary recipient animals. Data are mean values ± SEM of 10 or 5 IFNα-treated, 10 or 5 vehicle-treated (exp.1 or 2, respectively) and 5 nontreated (WT) mice. (B) Variations after 11 weeks of treatment (from exp. 1) in the percentages of cells from KI origin in BM, spleen, and blood. Are represented the early cell pools in BM (Lin-, MEP, CMP, GMP, LK, LSK, and SLAM cells determined as supplemental figure 4) (upper left) and CD11b/Gr1, B220, CD3 in BM (upper right), blood (lower left) and spleen (lower right). Data are mean values ± SEM, n = 3. (C) Secondary transplantation from exp. 2: variations in the hematological parameters and the percentages of KI-derived cells, analyzed by allele-specific PCR, of animals transplanted with BM from donors treated during 13 weeks with IFNα (continuous line) and vehicle (dashed line). Data are mean values ± SEM of 2 groups of 5 mice, each transplanted with BM from 2 different donors. Results from the secondary transplantation of exp. 1 are figured in supplemental Figure 11.
These results show that IFNα hampers JAK2V617F cell proliferation with normalization of most blood hematological values, except thrombocytopenia, according to disease severity.
To check if IFNα targeted the disease-initiating stem cells, we carried out secondary transplantation from 3 × 106 BM cells collected from IFNα- or vehicle-treated mice. As expected, mice transplanted with the vehicle-treated BM cells developed MPN with high hematocrit and WBC values (Figure 6C; supplemental Figure 11). In contrast, mice transplanted with the IFNα-treated BM cells developed normal hematocrit, platelet, and WBC values. We finally checked whether JAK2V617F-positive cells were still present in transplanted mice. From the IFNα-treated mice displaying the low severity disease, no cell from KI origin (CD45.2) was detected in the secondary recipients in contrast to vehicle-treated mice (supplemental Figure 11). Because we used CD45.2 recipients for IFNa-treated mice displaying the severe disease, we developed an allele-specific PCR assay to determine JAK2V617F allele burden in WBC. JAK2V617F was detected in WBC up to 7 weeks posttransplant and thereafter became undetectable (Figure 6C). In contrast, the level of JAK2V617F-WBC remained stable in mice transplanted with vehicle-treated BM cells from week 7 to 20 of survey.
These results show that long-term treatment by IFNα is able to alleviate JAK2V617F-derived MPN by suppressing long-term disease-initiating cells.
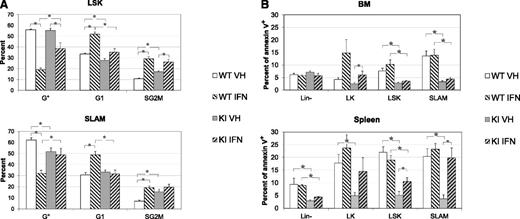
IFNα is known to induce antiproliferative and proapoptotic activities. To know if the effect of IFNα on JAK2V617F disease-initiating cells was mediated through cell cycle or apoptosis, we measured these parameters in LSK/SLAM cells from KI and WT littermate mice treated 3 days with IFNα (3 × 104IU) or vehicle. First, the results showed that LSK/SLAM cells from KI mice were more proliferative and less apoptotic than those from WT mice (Figure 7A-B; supplemental Figures 12 and 13). These properties may explain their amplification and proliferative advantage. Second, IFNα strongly induced the proliferation of WT LSK/SLAM, which, as previously reported,20 had an effect on KI LSK cell cycle but none on KI SLAM cell cycle. Overall, IFNα treatment induced WT SLAM cells to become as proliferative as KI SLAM cells. We noticed that IFNα increased Sca-I expression, as reported for IFNγ,31 and could bias our results, but we obtained similar results without including Sca-1 (that is dispensable) in SLAM cell characterization. Third, LSK and SLAM cells were clearly less apoptotic in KI than in WT mice. However, IFNα markedly induced apoptosis of spleen, but not BM, KI LK, and LSK/SLAM cells, and KI SLAM became as apoptotic as WT SLAM (Figure 7B; supplemental Figure 13).
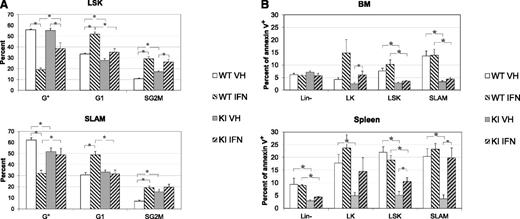
Effect of IFNα on cell cycle and apoptosis of LSK and SLAM cells from KI and WT mice. (A) Percentages of LSK (top) or SLAM (below) in G0 (Ki67Low/H333422n, quiescent), G1 (Ki67High/H333422n, activated), and S/G2/M (Ki67High/H333424n, cycling) phases after 3 days of in vivo treatment by vehicle or IFNα. Results show that KI SLAM are more proliferative than WT SLAM but that IFNα induced proliferation of WT LSK and SLAM cells, reducing the differences in cycle between WT and KI SLAM cells. (B) Percentage of apoptotic cells (annexin V) in BM (top) and spleen (bottom) of Lin-, LK, LSK, and SLAM cells after 3 days of in vivo treatment by vehicle or IFNα. Results showed that BM and spleen LSK and SLAM cells are less apoptotic in KI mice than in WT mice and that IFNα slightly increased apoptosis, especially of spleen KI cells. *P ≤ .05 with the 2-tailed unpaired Student t test. Both cycle and apoptosis results are mean values ± SEM, n = 6, of 2 independent experiments each including 3 IFNα-treated and 3 VEH-treated WT and KI mice around 3 months of age.
Effect of IFNα on cell cycle and apoptosis of LSK and SLAM cells from KI and WT mice. (A) Percentages of LSK (top) or SLAM (below) in G0 (Ki67Low/H333422n, quiescent), G1 (Ki67High/H333422n, activated), and S/G2/M (Ki67High/H333424n, cycling) phases after 3 days of in vivo treatment by vehicle or IFNα. Results show that KI SLAM are more proliferative than WT SLAM but that IFNα induced proliferation of WT LSK and SLAM cells, reducing the differences in cycle between WT and KI SLAM cells. (B) Percentage of apoptotic cells (annexin V) in BM (top) and spleen (bottom) of Lin-, LK, LSK, and SLAM cells after 3 days of in vivo treatment by vehicle or IFNα. Results showed that BM and spleen LSK and SLAM cells are less apoptotic in KI mice than in WT mice and that IFNα slightly increased apoptosis, especially of spleen KI cells. *P ≤ .05 with the 2-tailed unpaired Student t test. Both cycle and apoptosis results are mean values ± SEM, n = 6, of 2 independent experiments each including 3 IFNα-treated and 3 VEH-treated WT and KI mice around 3 months of age.
These results suggest that IFNα eradicates JAK2V617F disease-initiating cells in long-term treatment by increasing WT HSC cell cycle and KI HSC apoptosis, suppressing overall the proliferative advantage of KI over WT HSC.
Discussion
In this study, we show that endogenous expression of JAK2V617F in mouse HSCs induces a progressive amplification of early hematopoietic cells and provides to HSCs a strong competitive advantage over normal ones that could be explained by their high proliferative and low apoptotic status. Furthermore, long-term IFNα treatment suppresses disease progression by targeting JAK2V617F-expressing disease-initiating cells. Overall, this study suggests that JAK2V617F is capable of driving MPN emergence by itself from a low number of HSCs and that IFNα hampers this ability.
We previously described a constitutive JAK2V617F KI.10 In this report, a conditional KI with restricted expression in hematopoietic and some endothelial cells24 also generated a 2-stage MPN-mimicking human PV evolving into secondary myelofibrosis but with lower/more delayed splenomegaly/leukocytosis and polycythemia than our constitutive KI. This may be due to the late JAK2V617F gene expression controlled by the Vav promoter during fetal but not embryo life.32 All JAK2V617F Retroviral,5-7 TG,8 and KI9-12 mouse models have described the occurrence of MPN, emphasizing the responsibility of the mutation in this disorder. However, the phenotype of these models often differs. Studies have shown that the mutated/WT JAK2 ratio is crucial in determining disease diversity through various levels of RBC and platelet counts.5-8 However, it is not yet well understood why heterozygous expression of mouse JAK2V617F in all KI models induces a PV-like disease, one without thrombocytosis,11 whereas heterozygosity is usually associated with ET in humans. An ET-like disease is observed only in the heterozygous KI using the human gene.12
The effect of JAK2V617F on the amplification of late mature and maturing myeloid cells is well established in mice5-11 as in humans.18,33 In contrast, the effect of JAK2V617F on the amplification of early hematopoietic cells remains a matter of controversy.17 Our study shows that JAK2V617F increases in 6-month-old mice the percentage of LSK/SLAM cells by 5-/2-fold and 6-fold in marrow and spleen, respectively. These phenotypically defined cell pools are the most HSC-enriched compartments.29 Taking into account the BM and spleen cellularity, we measured a 7- or 5-fold expansion in the total numbers of LSK or SLAM cells in these mice, not including the contribution of the extra-medullary hematopoiesis evidenced in these animals. Interestingly, the pool of HSCs is quasi-normal at 1 month and expands only with age, while more mature cells amplify precociously. Our study also demonstrates the ability of JAK2V617F-expressing HSCs to compete with normal HSCs in transplantation settings with JAK2V617F-expressing blood granulocytes and tissue Lin-, LSK, and SLAM cells rapidly prevailing upon normal ones. Our results are compatible with 2 other KI models reporting an increase in BM LSKCD34-Flk2- cells34 and a competitive advantage of JAK2V617F-expressing HSCs recorded after 1 year of competitive transplantation.35 In contrast, others reported a functional impairment of HSCs by JAK2V617F,12 which could be related to the use of a human JAK2V617F gene. Overall, murine models indicate that JAK2V617F provides a clonal advantage to HSCs. Our study further shows that ∼30 JAK2V617F SLAM cells are sufficient to generate MPN with complete penetrance, which further emphasizes the sole role of JAK2V617F in this model and is compatible with a mathematic model suggesting that clonal dominance would need more than a single transformed cell to appear in a mouse lifetime.36 We also demonstrated that KI SLAM cells are less apoptotic and more proliferative than normal SLAM cells. This result may explain the proliferative advantage and amplification of KI over WT HSCs. The use of JAK2 by cytokines involved in HSC regulation, such as TPO,37,38 granulocyte colony stimulating factor,39 or IFNγ,31 may explain this result. This study shows no or very delayed lymphoid cell amplification by JAK2V617F, which emphasizes the mild role of JAK2 in lymphocyte signaling or suggests that amplified SLAM cells are myeloid biased.40 Intriguingly, amplification of KI SLAM cells in vehicle-treated primary recipients did not result in the same amplification of KI donor cells in secondary hosts. This result suggests that the amplification of SLAM cells by JAK2V617F does not correlate with HSC activity. Similar results were reported by Mullally et al,41 indicating that further experiments are needed to precisely determine the HSC properties of SLAM cells amplified by the mutation, with the caveat that high proliferative status may compromise their long-term self-renewal capacities.
In ET and PV patients, most studies agreed on the fact that the percentage of JAK2V617F-positive cells is lower in progenitor cells than in differentiated stages. In contrast, an amplification of JAK2V617F-positive cells was identified in progenitor and HSC of PMF or heterozygous PV patients,18,33,42 that may present more evolved forms of disease. Therefore, as shown by this study, JAK2V617F might give only a subtle advantage to HSCs that can be detected after several months in mice but only after several years in humans. As previously predicted,18,19 amplification of heterozygous JAK2V617F HSCs could also explain why heterozygosity leads to PV in mice but only to ET in humans unless additional molecular events, such as TET2 mutations, accelerate the upstream amplification of heterozygous cells. This hypothesis is corroborated by the association between an ET-like phenotype and the lack of early amplification in our young KI mice or KI mice using human JAK2V617F.12 In humans, the presence of other mutations affecting TET2,19 DNMT3a,16 or ASXL1 might not only give a further opportunity to the clone to develop the disease but also modify the phenotype or accelerate disease evolution. However, the possibility remains that human JAK2V617F is less functional, at least in a mouse context, than its mouse homolog and that these mutations become mandatory for disease emergence itself.
JAK2 inhibitors are currently tested in MPN patients and ruxolitinib was approved for use in the treatment of myelofibrosis.43 These drugs have essentially demonstrated palliative virtues with limited effects on the selective reduction of JAK2V617F-positive cells in human as in mice.11,44 In contrast, IFNα treatment has demonstrated hematological and molecular responses, including complete molecular responses in PV patients.22 Therefore, we tested the efficacy of IFNα in competitive transplantation models that included normal hematopoiesis and 2 different levels of JAK2V617F disease burden. IFNα rapidly reduced platelet and WBC counts and corrected erythrocytosis and splenomegaly according to disease severity. This is in agreement with previous studies in normal mice showing that IFNα impaired lymphopoiesis45 as well as myelopoiesis,46 inducing anemia, leukopenia, and thrombocytopenia.
Prolonged IFNα exposures (11-13 weeks) were used, because it seems an important factor for complete molecular responses in humans.22 Development of thrombocytopenia was observed. It has been attributed in humans to inhibition of platelet production by megakaryocytes.47 Most interestingly, the treatment targeted granulocytes but also SLAM JAK2V617F cells that were considerably reduced at the end of treatment. Secondary transplantations using treated marrow showed that disease developed normally from vehicle-treated cells but not from IFNα-treated cells. This result indicated that IFNα treatment suppressed JAK2V617F HSC amplification and proliferative advantage. This finding may give the opportunity to understand the mechanism of action of this drug that is largely unknown. Our results show that IFNα promoted cycling of normal stem cells, as previously reported,20,21 but did not further increased proliferation of JAK2V617F-SLAM cells. The reason could be that JAK2V617F already maximally stimulates the JAK/STAT signaling pathway that the IFNα receptor uses to promote HSC proliferation. However, cycling induced through the IFNα receptor and through other receptors31,37-39 using JAK2V617F may have distinct/antagonist effects31 on HSC maintenance (self-renewal or differentiation). It is also conceivable that increased signaling may be deleterious for HSCs, leading to loss of function, apoptosis, and finally exhaustion. Indeed, IFNα also increased apoptosis of SLAM cells in the spleen, and this effect may be major in reducing splenomegaly and suppressing neoplastic proliferation in a location that mostly contributed to its amplification. Among other mechanisms possibly involved, IFNα may promote an antitumor immune response through the stimulation of central memory T cells and activated dendritic cells.48,49 In humans, IFNα was shown to eradicate JAK2V617F cells without affecting TET2-mutated cells,50 suggesting a mechanism of action that is related to JAK2 signaling. Altogether, our results are in part similar to those recently obtained by Mullally et al41 in KI mice by showing that IFNα targets JAK2V617F HSCs, but our results differ in that the mechanism of IFNα is not yet clearly related to an entry of JAK2V617F HSCs in cell cycle and their subsequent exhaustion.
In conclusion, our study demonstrates that JAK2V617F is self-sufficient for HSCs to drive MPN in mice. Additional mutations may not be necessary to promote clonal emergence of MPN. The fact that IFNα alleviates the disease in mice offers the opportunity to understand the mechanism of action of this drug.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylvie Jacquot and Marie-Christine Birling from the Mouse Clinical Institute (ICS-MCI), Illkirch, France, for establishing the conditional KI mouse line; Dr Warren Alexander from the Walter Elisa Hall Institute, Melbourne, Australia, for the VavCre mice; the staff of the animal facilities of the IGR directed by Patrick Gonin, Philippe Rameau, and Yann Lecluse for cell sorting experiments; and Olivia Bawa for the histology and Françoise Wendling for critically reviewing the manuscript.
This work was supported by grants from Inserm, IGR, Institut National Contre le Cancer, and Cancéropôle Ile-de-France and by funding from La Ligue Nationale Contre le Cancer (labeled team 2009). S.H. had fellowships from the Ministère de la Recherche et de la Technologie and la Ligue Nationale Contre le Cancer. C.M. is a Fondation de France recipient.
Authorship
Contribution: S.H., C.L., M.C., and C.M. performed research and analyzed data; J.-L.V. designed experiments, performed research, analyzed data, and wrote the paper; and W.V. and E.S. designed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Luc Villeval, Inserm U1009, 114 rue Edouard Vaillant, Institut Gustave Roussy, PR1, 94805 Villejuif Cedex, France; e-mail: villeval@igr.fr.