Abstract
Despite continued achievements in antithrombotic pharmacotherapy, difficulties remain in managing patients at high risk for both thrombosis and hemorrhage. Utility of antithrombotic agents (ATAs) in these settings is restricted by inadequate pharmacokinetics and narrow therapeutic indices. Use of advanced drug delivery systems (ADDSs) may help to circumvent these problems. Various nanocarriers, affinity ligands, and polymer coatings provide ADDSs that have the potential to help optimize ATA pharmacokinetics, target drug delivery to sites of thrombosis, and sense pathologic changes in the vascular microenvironment, such as altered hemodynamic forces, expression of inflammatory markers, and structural differences between mature hemostatic and growing pathological clots. Delivery of ATAs using biomimetic synthetic carriers, host blood cells, and recombinant fusion proteins that are activated preferentially at sites of thrombus development has shown promising outcomes in preclinical models. Further development and translation of ADDSs that spare hemostatic fibrin clots hold promise for extending the utility of ATAs in the management of acute thrombotic disorders through rapid, transient, and targeted thromboprophylaxis. If the potential benefit of this technology is to be realized, a systematic and concerted effort is required to develop clinical trials and translate the use of ADDSs to the clinical arena.
Introduction
Thrombosis is a key event in the pathogenesis of myocardial infarction, venous thromboembolism (VTE), stroke, and other ischemic conditions that comprise the most common and growing cause of morbidity and mortality in industrialized communities. Development of antithrombotic agents (ATAs) is a global medical priority. ATAs may be subdivided conceptually into antiplatelet agents, anticoagulants, and fibrinolytics. Therapeutics within each category differ with respect to mechanism of action, time to onset, duration of effect, route of administration, and ease of monitoring and reversal (supplemental Table 1 on the Blood website).
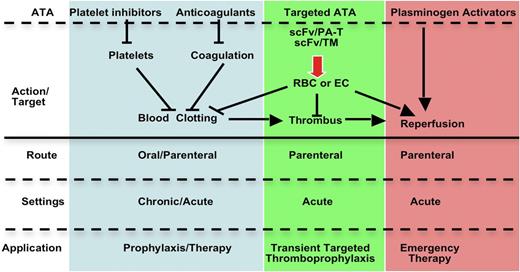
Traditionally, antiplatelet agents and anticoagulants have been perceived primarily as preventing clotting, whereas fibrinolytics have been viewed as rescue therapy in acute settings (supplemental Figure 1). However, many ATAs have dual preventive and therapeutic effects in acute settings due to the dynamic nature of clots, which may involve repetitive cycles of clot accretion, maturation, and dissolution. For example, anticoagulants are often used for treatment and prevention, due to their ability to both inhibit thrombus growth and to enhance endogenous fibrinolysis.
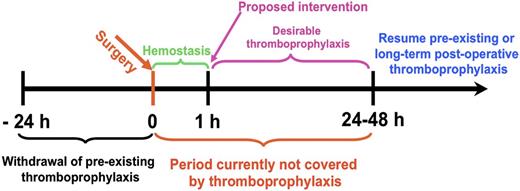
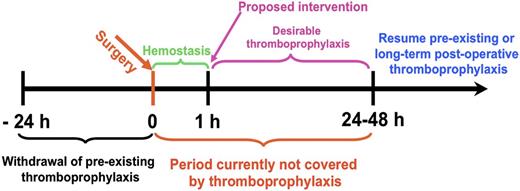
Although many ATAs are in clinical use, there continues to be a need to optimize efficacy and safety, especially in patients at high risk of bleeding and thrombosis. Narrow therapeutic indices complicate management of patients with commonly encountered clinical scenarios, including the prevention of venous thrombosis in high-risk surgical patients, such as those with trauma or cancer (Figure 1). Current approaches to thromboprophylaxis in such patients, although successful, necessitate a careful and oft-times difficult balance between the estimated risk of thrombosis with drug interruption and the risk of bleeding due to premature resumption of thromboprophylaxis.1-3 Stroke and delayed onset cognitive dysfunction attributed to thromboemboli remain a serious and increasingly recognized late complication of surgery. The risk of causing or accentuating bleeding also constrains the use of plasminogen activators (PAs) in patients with submassive pulmonary embolism and thrombotic stroke. In comparison with recent successes in the development of novel antiplatelet agents and anticoagulants, there have been fewer advances in fibrinolytic therapy since the introduction of tissue plasminogen activator (tPA). However, even partial preservation of perfusion affected by obstructing thrombi may suffice to prevent ischemic tissue injury. Therefore, it is not surprising that research in the use of advanced drug delivery systems (ADDSs) has mainly focused on fibrinolytic therapy.
Transient targeted thromboprophylaxis (TTT) for prevention of postsurgical thrombosis. Thromboprophylaxis is especially problematic in the early postoperative period. Thrombosis is a common complication of the trauma, inflammation, and immobility that accompany surgery, and thrombi are prone to recur or extend within hours of fibrinolysis, necessitating protracted activity. However, the risk of bleeding may preclude intervention for hours to days. Fibrinolytics are not used for prophylaxis because they are rapidly inactivated and cleared from the blood, necessitating use of high and unsafe doses to maintain activity. A rapid transient intervention that spares hemostatic clots, but prevents subsequent development of occlusive thrombosis elsewhere would fill a clear void in clinical management. This is but one of many settings in which patients at known imminent risk of thrombosis would benefit from TTT. An ideal agent for TTT would have an immediate onset of action that is of sufficient duration to prevent occlusive thrombi from forming without affecting preexisting fresh hemostatic clots (eg, those formed in the wound within ∼20-30 minutes of uncomplicated surgery) and without causing off-target toxicity.
Transient targeted thromboprophylaxis (TTT) for prevention of postsurgical thrombosis. Thromboprophylaxis is especially problematic in the early postoperative period. Thrombosis is a common complication of the trauma, inflammation, and immobility that accompany surgery, and thrombi are prone to recur or extend within hours of fibrinolysis, necessitating protracted activity. However, the risk of bleeding may preclude intervention for hours to days. Fibrinolytics are not used for prophylaxis because they are rapidly inactivated and cleared from the blood, necessitating use of high and unsafe doses to maintain activity. A rapid transient intervention that spares hemostatic clots, but prevents subsequent development of occlusive thrombosis elsewhere would fill a clear void in clinical management. This is but one of many settings in which patients at known imminent risk of thrombosis would benefit from TTT. An ideal agent for TTT would have an immediate onset of action that is of sufficient duration to prevent occlusive thrombi from forming without affecting preexisting fresh hemostatic clots (eg, those formed in the wound within ∼20-30 minutes of uncomplicated surgery) and without causing off-target toxicity.
A fundamental limitation shared by all current ATAs is the inability to prevent or reverse thrombosis without interfering with hemostasis. ADDSs have the potential to help overcome this limitation and thereby broaden the therapeutic window of antithrombotic interventions4 through 2 key, often intertwined, functions: (1) prolonging the circulation time and minimizing the undesirable drug/host interactions en route to the therapeutic site and (2) spatiotemporally localizing drug action.5 In this article, we briefly review using ADDSs for ATA.
Prolongation of ATA half-life and intravascular activity
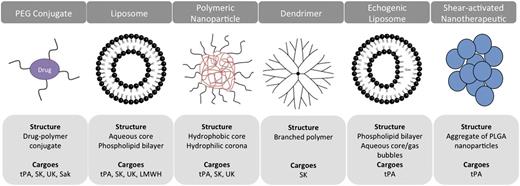
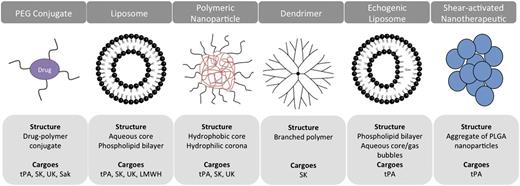
Multiple approaches have been devised to extend the half-life and activity of ATAs in the bloodstream, including polymer coating and the use of drug carriers. Most carriers are artificially assembled supramolecular structures composed of biocompatible elements, with sizes ranging from tens of nanometers to a few microns (Figure 2).6-8
ADDSs for ATA. The schematics present examples of classes of carriers tested for delivery of ATA, their principal structure, and PA cargoes. Synthetic carriers vary in size from few nanometers to several microns, shape, stability, and principle of drug loading and release. Liposomes and PEG represent the 2 most intensively studied ADDS for various ATAs including anticoagulants, platelet antagonists, and listed fibrinolytics.
ADDSs for ATA. The schematics present examples of classes of carriers tested for delivery of ATA, their principal structure, and PA cargoes. Synthetic carriers vary in size from few nanometers to several microns, shape, stability, and principle of drug loading and release. Liposomes and PEG represent the 2 most intensively studied ADDS for various ATAs including anticoagulants, platelet antagonists, and listed fibrinolytics.
Stealth polyethylene glycol coating, liposomes, and polymeric carriers
The first approach to prolong the half-life of ATAs involved conjugation with polyethylene glycol (PEG). PEG chains form a hydrated shell that enhances drug solubility and limits renal filtration, cellular uptake, proteolytic degradation, and immunogenicity (“stealth” ADDSs, an approach that has proven effective for some biotherapeutics).9 PEGylation of tPA,10 streptokinase (SK),11 urokinase (uPA),12 and staphylokinase (Sak)13,14 has been tested extensively. PEG-PA conjugates prepared with an optimal extent of protein modification exhibit a prolonged circulation time10-12,14 and minimal loss of activity.10 In a phase IIa trial, PEG-Sak had therapeutic efficacy similar to tPA, but the study revealed hemorrhagic complications.13
Liposomes, spherical vesicles with diameters ∼100 to 250 nm composed of a phospholipid bilayer (that may contain PEGylated phospholipids) surrounding an aqueous core, are used clinically to deliver chemotherapeutic agents.5 PEG liposomes protect ATAs from degradation,15 reduce antigenicity,16 and increase plasma half-life.17 ATAs encapsulated in PEG liposomes showed encouraging results in vitro and, to a more limited extent, in animal models. For example, liposomal PAs provided superior reperfusion vs free PAs in rabbit models of jugular vein18 and carotid artery19 thrombosis.
Interactions with cells and lipoproteins, as well as the intense PEGylation needed for a strong stealth effect, destabilize liposomes, limiting their lifetime in the bloodstream. More stable carriers based on amphiphilic polymers (frequently PEG block copolymers) have also been tested to extend the circulation time of ATAs. Examples include chitosan/poly(lactic-co-glycolic acid) nanoparticles for tPA, glycol chitosan/PEG nanogels for uPA, as well as polyglycerol and poly(amido amine) dendrimers for SK.20-23 In a canine model of coronary artery thrombosis, SK encapsulated within PEGylated particles caused faster reperfusion than free SK.24 However, in vivo data for polymeric carriers are even more limited than for PEGylated and liposomal ATAs.
These approaches prolong the circulation of ATAs on par with recombinant tPA variants (T1/2 in blood ∼5 minutes for tPA vs ∼20-40 minutes for Reteplase and the more potent PAI-1–resistant Tenecteplase),25 which have not provided a decisive therapeutic advantage. Additional studies are needed to determine the factors that contribute to clot resistance in vivo, including poor perfusion of occluded vessels, local high levels of PAI-1, clot retraction, and high fibrin affinity limiting clot penetration, among others.26 Enlargement of ATAs by a carrier (eg, from a few nanometers up to hundreds) further impedes clot permeation. The mechanism by which encapsulated ATAs could interact with fibrin and distinguish between occlusive vs hemostatic thrombi is unclear. Additionally, the prolongation of half-life is still not sufficient for prophylactic administration, which might enable ATAs to be delivered to the interior of nascent clots, thereby overcoming permeability issues.
ATA carriers sensitive to ultrasound and hydrodynamic forces
One approach toward circumventing these problems is to release ATAs from carriers at the intended therapeutic site. Echogenic liposomes (ELIPs), which contain both gas and fluid in the core, can be used for drug delivery and ultrasound imaging. Locally applied ultrasound facilitates reperfusion of thrombotic vessels in animals injected with tPA-loaded ELIPs,27,28 most likely by accelerating tPA release and disintegrating clots directly, enhancing drug permeation. PEG-gelatin nanocarriers also prolonged the circulation of tPA and released it in an ultrasound-responsive fashion, improving recanalization of occlusive clots in rabbits.29 Further refinements of the ELIP approach are warranted, as potent ultrasound may inactivate PAs30 and cause vascular damage. Some clots may not be amenable to this approach due to unknown location or risk of hemorrhage.
To avoid the need for an external mechanism to release ATAs, “smart” carriers sensitive to hemodynamic changes have recently been designed (supplemental Figure 2). These shear-activated nanotherapeutics (SA-NTs)31 represent micron-size (∼4 μm diameter) aggregates of tPA-coated polymeric nanoparticles (∼200 nm diameter) that are relatively stable under normal flow (10-30 dyne/cm2) but dissociate into nanoparticles at the elevated shear stress levels encountered in areas of arterial stenosis (>100 dyne/cm2). Nanoparticles experience lower drag forces than their parental micron particles and accumulate in affected vessels, thereby delivering the ATA. In a mouse model of thrombotic arterial stenosis, SA-NTs prolonged the time to vessel occlusion from ∼10 to ∼30 minutes in vivo and cleared pulmonary emboli in an ex vivo model at a 100-fold lower dose than free tPA. Lenticular lipid-based vesicles that show shear stress–induced drug release have also been developed but have not yet been evaluated in vivo.32
Nonspherical carriers imitating the hemodynamic properties of platelets33 or red blood cells (RBCs)34,35 circulate in model microfluidic systems and animals for extended periods of time, exceeding the limits of traditional nanocarriers.34 Elongated PEG polymeric filomicelles that align with blood flow and thereby avoid collisions with vascular cells have circulation times on the order of days as opposed to hours as seen with spherical PEG-coated carriers.36 This may allow them to integrate into growing thrombi. For example, when coated with a recombinant VWF-A1 fragment that targets the GPIbα region on activated platelets, “synthetic platelets” were incorporated into clots.33 This is a new area of research, and the in vivo behavior and therapeutic efficacy of ATA-loaded particles remain to be tested. Challenges of using such polymeric carriers include inactivation of enzymatic drugs during loading and the need for substrate permeability or controlled release.37 Encapsulation of active enzymes in filomicelles has been reported,38 which provides hope they can find utility for delivering ATAs. These studies also point toward exploring the natural carriers, blood cells, which inspired their development.
ATA delivery by erythrocytes: transient targeted thromboprophylaxis
RBCs (∼6-μm discs with a circulation time of ∼120 days in humans) have many features of ideal carriers for drugs that act in the bloodstream, especially when sustained action is needed.39-41 Drugs can be encapsulated within isolated RBCs followed by resealing and injection.42 Promising results from transfusion of RBC-encapsulated drugs in neurological, oncological, and other diseases have been reported.41,43-46 Biocompatible coupling of ATAs to the surface of carrier RBCs represents a preferable alternative to encapsulation.47
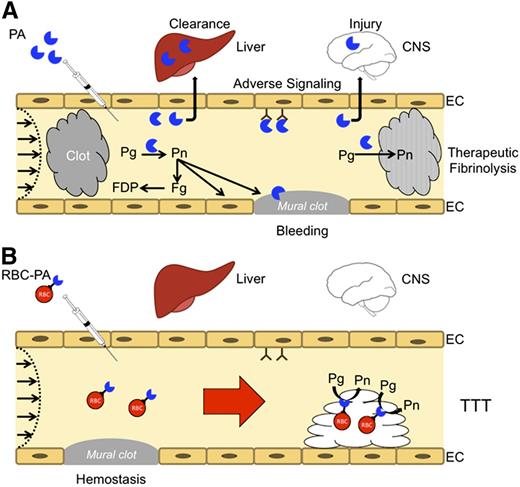
Once clots are established, they become progressively impermeable to RBCs. Although this feature negates the utility of RBC carriers for therapeutic thrombolysis, it also provides a key safety feature for transient targeted thromboprophylaxis (TTT). In theory, incorporation of PAs within the interior of nascent thrombi will arrest clot propagation and dissolve clots from within more effectively than PAs permeating the clot from the surface. If feasible, anticipatory delivery of PAs within nascent thrombi might prevent vascular occlusion in patients at imminent risk of thrombosis without lysing hemostatic clots. Although the inherent limitations of PAs discussed above preclude their use in thromboprophylaxis, coupling PAs to RBCs surmounts these problems (Figure 3).
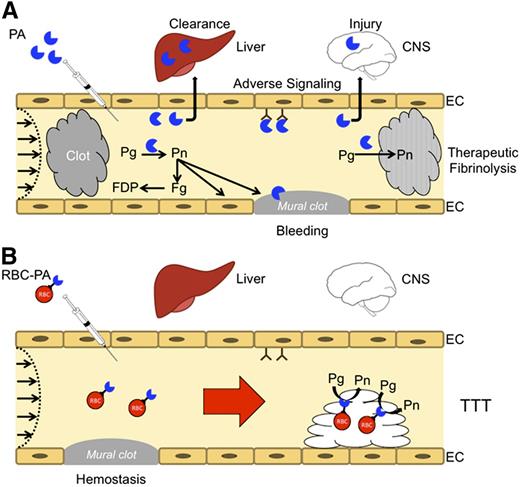
Coupling PAs to carrier RBC may convert risky fibrinolytic therapy into safe transient thromboprophylaxis. (A) PAs are used in the emergency setting for reperfusion. High doses are needed to compensate for rapid inactivation by PAI-1 and clearance by the liver and other organs. PAs diffuse into hemostatic mural clots, as well as occlusive pathological clots, increasing the risk of bleeding, and into tissues, such as the CNS, where they cause toxicity. Therapeutic doses can deplete fibrinogen and plasminogen increasing the risk of bleeding while reducing fibrinolytic potential. Only a small proportion of the residual circulating PA reaches and penetrates the interior of clots. (B) RBC-coupled PAs avoid rapid clearance, circulating for many hours to days without damaging carrier RBCs. The large size and hemodynamic factors prevent access to preexisting hemostatic clots, limit extravasation into tissues, and block interaction with cellular receptors such as uPAR (CD87) and integrins that may initiate deleterious intravascular signaling cascades. RBC-coupled PAs are incorporated within nascent intravascular clots formed after treatment, which they lyse from within, rapidly restoring flow and preventing ischemia at doses that are orders of magnitude less than free fibrinolytics.
Coupling PAs to carrier RBC may convert risky fibrinolytic therapy into safe transient thromboprophylaxis. (A) PAs are used in the emergency setting for reperfusion. High doses are needed to compensate for rapid inactivation by PAI-1 and clearance by the liver and other organs. PAs diffuse into hemostatic mural clots, as well as occlusive pathological clots, increasing the risk of bleeding, and into tissues, such as the CNS, where they cause toxicity. Therapeutic doses can deplete fibrinogen and plasminogen increasing the risk of bleeding while reducing fibrinolytic potential. Only a small proportion of the residual circulating PA reaches and penetrates the interior of clots. (B) RBC-coupled PAs avoid rapid clearance, circulating for many hours to days without damaging carrier RBCs. The large size and hemodynamic factors prevent access to preexisting hemostatic clots, limit extravasation into tissues, and block interaction with cellular receptors such as uPAR (CD87) and integrins that may initiate deleterious intravascular signaling cascades. RBC-coupled PAs are incorporated within nascent intravascular clots formed after treatment, which they lyse from within, rapidly restoring flow and preventing ischemia at doses that are orders of magnitude less than free fibrinolytics.
RBC carriers were first devised for PAs.48,49 Studies in mice, rats, and pigs have shown that conjugation of PAs to RBCs generates a biocompatible RBC/PA complex that does not lyse hemostatic clots formed as recently as 10 minutes after surgery that are readily lysed by free PA.49,50 The circulation of RBC/PA in the blood is several orders of magnitude longer than PA (many hours vs minutes),50 which allows inclusion of prophylactically injected RBC/PA within nascent intravascular clots followed by rapid lysis and reperfusion, in settings where a 10-fold higher dose of soluble PA is ineffective.49-51 As clots mature, they generate less thrombin and undergo physical retraction, which markedly reduces penetration by RBCs and even by tPA. Moreover, hemodynamic forces divert circulating RBCs from mural clots. For these and other reasons, RBC/PA show selectivity toward lysing nascent clots forming in the lumen compared with preformed clots.
The RBC glycocalyx attenuates inactivation of coupled tPA by PAI-1,52 without affecting its fibrin affinity, which facilitates RBC/tPA entrapment in growing thrombi.51 Coupling urokinase to RBCs blocks its interaction with cellular uPA receptors and limits unwanted adhesion of RBC/PA to vessel walls.53 RBC/PA entrapped within growing clots rapidly form cavities that merge under flow to create patent channels that permit expedited perfusion by RBCs prior to extensive disintegration of clot structure.54,55
Intravenously injected RBC/PA rapidly lyse nascent cerebrovascular thrombi, leading to reperfusion, protection of brain tissue, and improved survival.56 In animal models of traumatic brain injury57 and intracranial hemorrhage,58 RBC/PA injected shortly after trauma attenuated secondary thrombosis and neuronal death seen in control animals.56-60 In contrast, soluble PA exacerbated CNS bleeding, neurotoxicity, and lethality. Further, RBC/tPA preserved cerebral vascular autoregulation disrupted by cerebral hypoxia in contrast to tPA, which increased cerebrovascular contractility and tissue ischemia.59 Finally, tPA aggravated inflammatory pathways and brain injury in pig models of global cerebral hypoxia and focal thrombotic ischemia, whereas RBC/tPA activated anti-inflammatory pathway(s) and attenuated brain injury.60 Therefore, coupling to RBC effectively switches the PA signaling profile in the CNS from injurious to protective, permitting a reduction in dosing and mitigating ischemia by enhancing clot lysis, while reducing deleterious intravascular and extravascular signaling.61
These RBC loading/transfusion studies show the potential advantages of prophylactic thrombolysis—a concept that might expand the current paradigm of fibrinolytic therapy.
Prolonging ATA circulation: challenges and perspectives
Artificial carriers designed to prolong half-life and improve delivery of ATAs are not yet ready for clinical use. Although each carrier has its own advantages and disadvantages (Table 1), none prolong the longevity of ATAs in the circulation sufficiently to capitalize on the dynamic nature of developing clots. Their therapeutic utility for fibrinolysis is impeded by the impermeability of maturing clots. Flow-sensitive nanocarriers may overcome some of these limitations in settings characterized by partial thrombotic occlusion and pathologically high shear stress, although their utility in low shear conditions may be more limited.
RBCs markedly prolong the circulation and limit the side effects of fibrinolytics sufficiently to envision their utility in thromboprophylaxis. RBC/tPA prevented venous and arterial thrombotic occlusions in animal models. The relative effectiveness of RBC vs platelet ATAs in preventing venous vs arterial thrombosis requires further study. Clinical heterogeneity in clot maturation and the attendant risk of bleeding and clotting will require extensive preclinical investigation to determine dosing and timing to optimize the benefit to risk ratio of any intervention.
Intravascular targeting of ATAs
A variety of affinity ligands including antibodies and their single chain variable fragments (scFvs), clot components, aptamers, and peptides have been used to target fibrinolytics and, to a lesser extent, anticoagulants.25,62 The most intensively investigated targets for delivering ATAs have been clotting factors, components of clots, blood components that may deliver a cargo to clots and endothelial surface determinants providing anchoring of cargoes in the vascular lumen (Table 2).63
Chemical conjugation of large ATA molecules such as heparin and proteins (eg, tPA) with these ligands generated products that vary in specific activity, molecular structure, valence, and size, which impedes industrial and clinical translation. Recombinant constructs fusing the drug with scFv fragments (supplemental Figure 3) are advantageous, because (1) binding of monovalent scFvs does not cross-link epitopes that could lead to cellular agglutination, activation, and endocytosis; (2) scFv fusions lack Fc fragment–mediated side effects; (3) modular formats support rapid synthesis of iterations of ATA fusions bearing different cargoes; (4) affinity of fusions can be optimized in vitro; and (5) industrial GMP production of homogeneous scFv fusions and techniques for scFv humanization minimizing the likelihood of immune reactions are established.64,65
The recombinant format of ATA fusions makes it amenable to introducing functionally advantageous mutations into the cargo. For example, uPA is synthesized as a single chain zymogen (scuPA) in contrast to tPA, which is constitutively active. Proteolysis of scuPA between Lys135 and Lys136 yields an ∼30-kDa zymogen, low-molecular-weight (lmw) scuPA that lacks the growth factor–like and kringle domains that bind to CD87/uPAR and other receptors on vascular cells and leukocytes, triggering potentially deleterious signaling pathways.66 Cleavage of scuPA or lmw scuPA by plasmin at Lys158-Ile159 yields enzymatically active two-chain molecules, tcuPA and lmw tcuPA,67 which produce plasmin.68
Insertion of cleavage sites for proteases involved in clotting (eg, thrombin and Factor Xa) creates a gradient for activating latent ATA cargoes emanating from the site of incipient thrombosis (Figure 4; supplemental Figure 3). For example, physiologic activation of scuPA by plasmin may not suffice to prevent thrombosis, and tcuPA is rapidly inactivated by plasma inhibitors. Thrombin also inactivates uPA by cleaving Arg156-Phe157, negating its effect at sites of active thrombosis. These problems can be circumvented by deleting Phe157 and Lys158 to yield a plasmin-resistant mutant activated by thrombin (uPA-T).69 This pro-drug is not activated by plasmin in vivo (thus avoiding systemic effects and premature inactivation), but engenders activation by thrombin locally at sites of nascent thrombosis within seconds of clotting. This mutant, scuPA-T, is thereby invested with a more favorable targeting profile.69
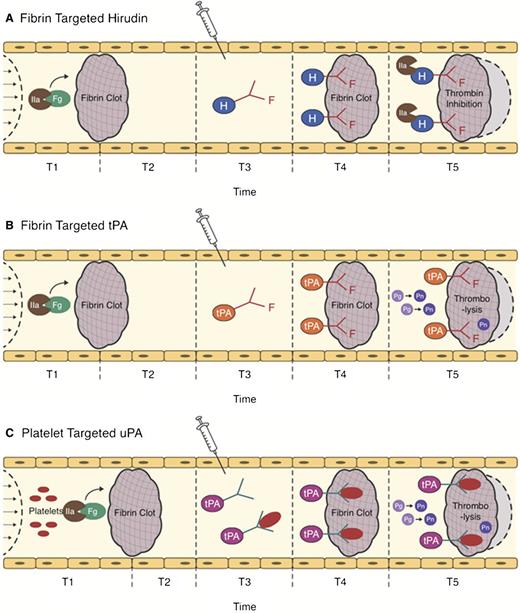
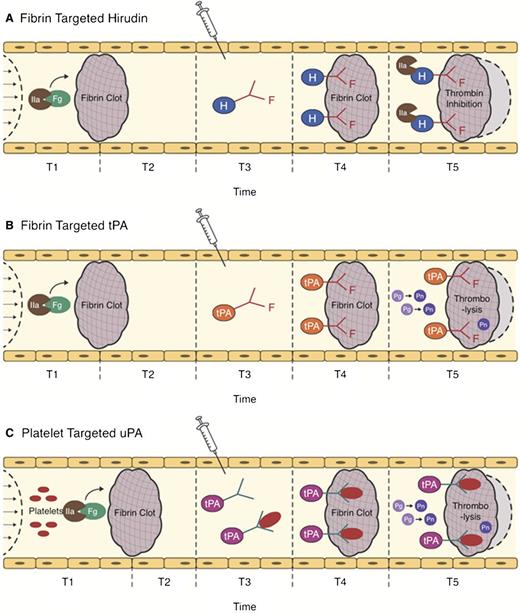
Strategies for ATA targeting. The action of clot-targeted scFv/ATA has been studied in animal models of arterial and venous thrombosis. ATA fusions or antibody conjugates injected in the vasculature shortly after thrombotic occlusion (T3) circulate for a limited period of time. Affinity to clot components enhances the fraction of injected ATA accumulating at the thrombotic site. (A-B) Fibrin-targeted ATAs bind to fibrin accessible at the clot surface and to additional fibrin depositing at the site of thrombosis in the course of clot growth and remodeling (light gray area surrounded by dash line). (C) ATAs bind to activated platelets accessible from the clot surface and in the remodeling clot, or to resting platelets (which may prolong circulation and provide continuous platelet-mediated delivery into secondary or remodeling clots). (A) Clot-associated anticoagulants (eg, hirudin) inhibit additional formation of thrombin, thus limiting thrombus growth, (B-C) whereas PAs facilitate dissolution of clots and foster reperfusion.
Strategies for ATA targeting. The action of clot-targeted scFv/ATA has been studied in animal models of arterial and venous thrombosis. ATA fusions or antibody conjugates injected in the vasculature shortly after thrombotic occlusion (T3) circulate for a limited period of time. Affinity to clot components enhances the fraction of injected ATA accumulating at the thrombotic site. (A-B) Fibrin-targeted ATAs bind to fibrin accessible at the clot surface and to additional fibrin depositing at the site of thrombosis in the course of clot growth and remodeling (light gray area surrounded by dash line). (C) ATAs bind to activated platelets accessible from the clot surface and in the remodeling clot, or to resting platelets (which may prolong circulation and provide continuous platelet-mediated delivery into secondary or remodeling clots). (A) Clot-associated anticoagulants (eg, hirudin) inhibit additional formation of thrombin, thus limiting thrombus growth, (B-C) whereas PAs facilitate dissolution of clots and foster reperfusion.
Targeting ATA to thrombi
The discovery of fibrin-specific epitopes absent in fibrinogen provided the rationale and tools for targeting ATAs to thrombi.70 Fibrin-targeted ATA conjugates, nanocarriers, and scFv fusions with thrombin-induced ATA activation and release (Figure 4) show enhanced antithrombotic therapy in animal models.71,72
Platelets have also been exploited as carriers for ATAs, especially for arterial clots.73 Ligands recognizing the conformational change in GPIIb/IIIa or P-selectin exposed on platelet activation might direct ATAs or ATA-loaded carriers to growing platelet-rich clots.74,75 For example, uPA conjugated to a monoclonal anti-GPIIb/IIIa scFv binds to platelets and platelet-rich thrombi and lyses arterial thrombi.76 Some of the conjugate’s activity might derive from blocking the fibrinogen receptor GPIIb/IIIa, like Abciximab.77 Platelet-targeted ATAs may accumulate in thrombi by binding to (1) resting platelets in the circulation with subsequent incorporation into growing clots and/or (2) platelets within clots themselves (Figure 4C). The contribution of the latter pathway needs to be better defined, for example, by tracing injected ATA derivatives in blood and thrombi.78
However, the conundrum of targeting ATAs to clot components is that their accessibility diminishes rapidly during thrombosis. Paradoxically, the higher the affinity of a ligand for fibrin, the greater is its retention on the clot surface and the less its penetration.79 However, clot-targeted ATAs have insufficient longevity for use as thromboprophylaxis. Finally, no existing ligand has been shown to distinguish pathological from hemostatic clots.
Targeting ATAs to circulating RBCs
ATA targeting to RBCs, ie, a logical extension of the principle described previously, helps circumvent many of these issues and bypasses the complexity, delays, and potential risks associated with ex vivo coupling to allogeneic RBCs. As a prototype, tPA conjugated with a monoclonal antibody to RBC glycoprotein complement receptor 1 bound rapidly to RBCs in the bloodstream and circulated safely for many hours after injection in mice, providing prophylactic thrombolysis without hemorrhagic side effects, similar to preformed RBC/tPA.80 Subsequently, a scFv/tPA fusion targeted to RBC glycophorin-A related antigen (GPA) was developed that bound safely to circulating RBC and had antithrombotic effects in mouse models of thrombosis qualitatively similarly to RBC/tPA and superior to tPA, with an additional advantage of wide range of dosing options (∼106 copies of GPA vs 2 × 103 complement receptor 1 copies per RBC, respectively).80-82
To enhance spatiotemporal control, the tPA cargo was replaced with lmw-scuPA in which the natural plasmin-sensitive activation site was replaced with a thrombin-sensitive site. Animal studies showed that the resultant scFv/uPA-T fusion (1) binds safely to RBCs after intravenous injection; (2) circulates as a RBC-bound pro-drug; (3) is activated by thrombin; and (4) provides effective and safe TTT with higher fidelity and efficacy than even scFv/tPA (Figure 5A).82
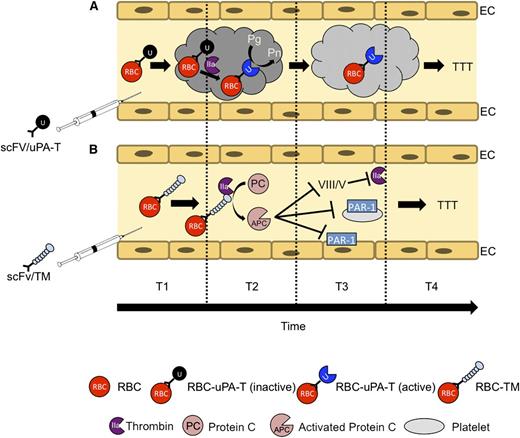
TTT by RBC-targeted fusion ATA pro-drugs. Targeted ATAs, including fibrinolytics such as PAs (tPA and uPA) and anticoagulants such as TM, bind to RBCs in the bloodstream and circulate for a prolonged time without damaging the cell carrier or causing other adverse effects typical of soluble drugs. Using RBC-targeted fusion constructs avoids the need for ex vivo loading and transfusion, thereby increasing clinical utility. Altering the mode of enzymatic activation from plasmin to thrombin enhances safety and localization. (A) scFv/uPA-T containing a variant of uPA sensitive to activation by thrombin exerts maximal fibrinolytic activity at sites of active clotting where the concentration of thrombin is greatest. (B) scFv/TM facilitates local conversion by thrombin of protein C into APC, which in turn inactivates activated coagulation factors Va and VIIIa and inhibits prothrombotic and proinflammatory signaling via protease activated receptors (PARs) on platelets and ECs, respectively.
TTT by RBC-targeted fusion ATA pro-drugs. Targeted ATAs, including fibrinolytics such as PAs (tPA and uPA) and anticoagulants such as TM, bind to RBCs in the bloodstream and circulate for a prolonged time without damaging the cell carrier or causing other adverse effects typical of soluble drugs. Using RBC-targeted fusion constructs avoids the need for ex vivo loading and transfusion, thereby increasing clinical utility. Altering the mode of enzymatic activation from plasmin to thrombin enhances safety and localization. (A) scFv/uPA-T containing a variant of uPA sensitive to activation by thrombin exerts maximal fibrinolytic activity at sites of active clotting where the concentration of thrombin is greatest. (B) scFv/TM facilitates local conversion by thrombin of protein C into APC, which in turn inactivates activated coagulation factors Va and VIIIa and inhibits prothrombotic and proinflammatory signaling via protease activated receptors (PARs) on platelets and ECs, respectively.
Fibrinolytics are not the only potential ATA cargo suitable for RBC carriage. For example, recombinant soluble thrombomodulin (sTM), a natural anticoagulant that shows encouraging clinical results,83 has a half-life in the circulation that can reach several days after subcutaneous injection. This can be extended many fold by targeting to RBCs, and the onset of its action can be expedited by intravenous administration of sTM fused with scFv anti-GPA. In animal models, RBC-targeted anti-GPA scFv/TM (1) binds safely to RBCs; (2) generates activated protein C (APC) from PC in the presence of thrombin; (3) has a half-life ∼100-fold longer than sTM after intravenous injection; and (4) prevents occlusive arterial and venous thrombi from forming at doses that are orders of magnitude lower than sTM.84 TM and APC have multifaceted antithrombotic and anti-inflammatory activities. RBC-targeted scFv/TM may find therapeutic and prophylactic utility in such intertwining pathologies (Figure 5B).
In summary, targeting to carrier RBCs might offer a preferred approach to deliver and locally activate antithrombotic pro-drugs within incipient thrombi while sparing even recent hemostatic clots that become impermeable to RBCs within minutes. Targeting scFv/uPA-T and scFv/TM to RBCs might provide TTT for patients at high risk of thrombosis in the early postsurgical period or with related conditions that combine acute risk of thrombosis with a heightened risk of hemorrhage.
Targeting ATAs to endothelial cells
Endothelial cells (ECs) use natural antithrombotic mechanisms to maintain blood fluidity, including synthesis of a heparan sulfate–rich glycocalyx that binds antithrombin (which inhibits thrombin), production of nitric oxide (which inhibits platelet activation), expression of tPA, and surface expression of TM (which diverts the procoagulant activities of thrombin to the antithrombotic functions of APC; Figure 6A). These mechanisms are suppressed by inflammation, shifting the local environment toward a prothrombotic phenotype.85 Targeting ATAs to the luminal surface of the endothelium is intended to compensate for these prothrombotic changes without compromising normal tissue responses to injury.
Endothelial Targeting of ATA. (A) Healthy ECs express antithrombotic activity, using a variety of antiplatelet, anticoagulant, and fibrinolytic mechanisms to maintain blood fluidity. (B) Thrombin-dependent therapeutics, including lmw-scuPA-T and TM, can be anchored to the luminal membrane of ECs using PECAM-targeted scFv antibody fragments. This approach has the potential to augment endogenous antithrombotic mechanisms, which developed through millions of years of evolution to prevent thrombosis without compromising normal tissue responses to injury.
Endothelial Targeting of ATA. (A) Healthy ECs express antithrombotic activity, using a variety of antiplatelet, anticoagulant, and fibrinolytic mechanisms to maintain blood fluidity. (B) Thrombin-dependent therapeutics, including lmw-scuPA-T and TM, can be anchored to the luminal membrane of ECs using PECAM-targeted scFv antibody fragments. This approach has the potential to augment endogenous antithrombotic mechanisms, which developed through millions of years of evolution to prevent thrombosis without compromising normal tissue responses to injury.
Transfection of endothelium with genes encoding ATAs provided a prototype for such intervention. For example, transfected baboon ECs expressing tPA or a membrane-anchored form of uPA inhibited formation of platelet- and fibrin-rich thrombi in vitro,86 as did jugular vein segments transduced to express TM prior to graft insertion.87 However, the challenge of applying gene therapy to acute settings limits its utility in managing thrombosis.
Conjugating ATAs to ligands that bind to the endothelial surface offers a potential alternative approach. Ideally, ATAs should anchor preferentially onto prothrombotic endothelium without cell injury or internalization of drug. For example, hirudin cross-linked to anti–E-selectin antibody binds to cytokine-activated ECs and inhibits thrombin in vitro88 but has limited therapeutic utility due to endocytosis in vivo.89 Likewise, an anti–angiotensin converting enzyme/tPA conjugate is highly effective at targeting the endothelium in vivo but is endocytosed and has little fibrinolytic utility.90-92
The endothelial adhesion molecules platelet-EC adhesion molecule (PECAM)-1 and intercellular adhesion molecule (ICAM-1) are attractive targets for ATA delivery, respectively, to normal (ie, prophylaxis) or activated (therapy) endothelium.93,94 Inhibition of their native functions may provide additional anti-inflammatory benefit. Although ECs internalize multivalent antibody conjugates targeting ICAM-1 and PECAM-1,95 they do not internalize monomolecular ligands.96 Accordingly, a series of ATAs fused with monovalent scFv fragments of PECAM-1 antibodies have been devised and tested in animal models. Prophylactic injection of lmw-scuPA fused with anti-PECAM scFv enhanced lysis of subsequently formed emboli in the pulmonary vasculature.97 Similarly, prophylactic injection of this fusion via the carotid artery resulted in the rapid lysis of thromboemboli in the cerebral vasculature and attenuation of cerebral edema, whereas its untargeted ATA counterpart exacerbated brain injury.98
PECAM-targeting of ATAs, which acts locally in a thrombin-dependent manner, has also been tested (Figure 6B). scFv/lmw-scuPA-T provided potent thromboprophylaxis against thrombin-induced pulmonary thrombosis, reduced pulmonary deposition of fibrin, and improved oxygenation in a model of focal pulmonary ischemia/reperfusion injury.99 Endothelial targeting of scFv/TM may provide even greater opportunities for enhanced anticoagulant and anti-inflammatory effects through interactions with the endothelial protein C receptor.100 Ongoing preclinical studies are focused on segregating the diverse activities of scFv/TM, including quenching of cytokines and generation of thrombin-activatable thrombolysis inhibitor, in addition to its antithrombotic activity.
Conclusion: challenges and opportunities
Emergency thrombolysis and thromboprophylaxis rely on the narrow margin between benefit and risk inherent in the use of existing ATAs. These drugs fail to discriminate between healthy and at-risk vasculature and distribute widely in the circulation. Recently developed ADDSs, designed to profit from altered hemodynamic forces and target accessibility, might be capable of sensing subtle differences between mural hemostatic and occlusive thrombi. Such ADDSs, including those using natural blood cells of the host as carriers, hold promise to enable TTT, a rapid and precise intervention capable of distinguishing pathological from hemostatic clots, and provide safe and effective management of acute thrombotic disorders not amenable to current treatment regimens (Figure 7).
ADDSs cover a new area of antithrombotic utility: TTT. Using ADDSs has the potential to provide ATA pro-drugs with an immediate onset (local activation) and a duration of action lasting hours to days, targeted carriers that capitalize on the dynamic nature of thrombi, and differences between preformed hemostatic and subsequently formed occlusive clots. This approach might extend the medical utility of fibrinolytic or anticoagulant ATAs.
ADDSs cover a new area of antithrombotic utility: TTT. Using ADDSs has the potential to provide ATA pro-drugs with an immediate onset (local activation) and a duration of action lasting hours to days, targeted carriers that capitalize on the dynamic nature of thrombi, and differences between preformed hemostatic and subsequently formed occlusive clots. This approach might extend the medical utility of fibrinolytic or anticoagulant ATAs.
By changing ATA delivery in a manner that concentrates effective doses in the vicinity of the target and provides a clinically appropriate temporal window, it might be possible to capitalize on advantages provided by the dynamic nature of clot initiation, propagation, and perseverance. Most likely, the clinical utility of targeted ATAs requiring parenteral administration will be limited to acute settings, such as in the Emergency Department or Interventional Radiology suite. Conditions that may be amenable to delivery of ATA (supplemental Figure 4) include non-ST segment elevation-AMI,101,102 submassive pulmonary embolism, and acute mesenteric or limb ischemia. TTT might also find utility in postsurgical patients (Figure 1), as a means to prevent postperfusion syndrome during cardiopulmonary bypass, and, perhaps, in patients suffering stuttering cerebral ischemia from recurrent in situ thrombosis or thromboembolism. Recombinant scFv/fusion proteins allow targeting of functional fragments of pro-drugs to sites of ongoing thrombosis using diverse carriers and targets (RBCs, endothelium, platelets, etc) and activation options. Biological drugs such as recombinant fusions have been incorporated into clinical care and nanocarriers have been tested to treat oncological diseases.
Despite substantial preclinical data indicating a potential role for ADDSs in the delivery of ATAs, enthusiasm must be tempered by several important factors. Targeted ATAs have not been tested in humans, and their translation to clinical use will be challenging. The animal models in which the majority of testing has occurred may not mirror important clinical features, especially the risk of bleeding. Clinical trial design is likely to be challenging due to the acute and unpredictable onset of the relevant thrombotic conditions. It might be difficult to find a comparison group of free drug in those settings in which we envision use of targeted ATAs, and current thromboprophylaxis poses too high a risk. At the same time, any new agent will have to show superior efficacy, safety, or another compelling benefit over existing therapy, at a time in which multiple new small-molecule ATAs are vying to replace older agents in routine clinical use. As with any new biotherapeutic, production costs may be higher than they are for synthetic ATAs and, at least initially, the regulatory landscape may be complex. However, if ATAs delivered via ADDSs can demonstrate a meaningful benefit in patient outcome in well-chosen clinical settings, this approach is likely to overcome these hurdles and attract considerable interest from physicians and pharmaceutical companies alike.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL087036, HL090697, and HL091950.
Authorship
Contributions: C.F.G., M.D.H., R.C., D.B.C., and V.R.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vladimir Muzykantov, University of Pennsylvania, Perelman School of Medicine, Department of Pharmacology and Center for Targeted Therapeutics and Translational Nanomedicine, TRC10-125, 3600 Civic Center Blvd, Philadelphia, PA 19104; email: muzykant@mail.med.upenn.edu.
References
Author notes
C.F.G. and M.D.H. contributed equally to this work.