In this issue of Blood, Dhodapkar et al1 present the first US cooperative group trial in asymptomatic monoclonal gammopathies that prospectively examines state-of-the-art laboratory, genomic, and imaging as potential predictors for progression to active multiple myeloma.
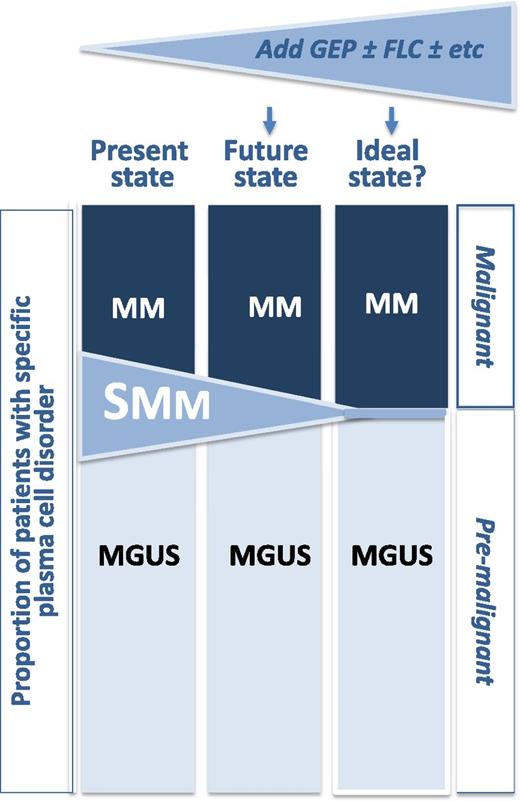
Proportions of patients with specific plasma cell disorder. By adding additional variables like GEP, serum immunoglobulin FLC, and perhaps other variables like MRI and M-protein size, and bone marrow plasmacytosis, the transitional category of SMM may shrink, allowing for more refined definitions of premalignant (MGUS) and malignant (active MM) plasma cell disorders.
Proportions of patients with specific plasma cell disorder. By adding additional variables like GEP, serum immunoglobulin FLC, and perhaps other variables like MRI and M-protein size, and bone marrow plasmacytosis, the transitional category of SMM may shrink, allowing for more refined definitions of premalignant (MGUS) and malignant (active MM) plasma cell disorders.
This observational trial included 179 smoldering multiple myeloma (SMM) and 152 monoclonal gammopathy of unknown significance (MGUS) patients and illustrates both the feasibility and challenges of such a study: 331 eligible patients were accrued, but only 26% of MGUS patients and 49% of SMM had gene expression profiling (GEP) performed, and only 42% of MGUS and 51% of SMM patients had magnetic resonance imaging (MRI) completed.
All GEP-defined molecular subtypes seen in active multiple myeloma (MM) were present in MGUS and SMM, but only the proliferation subtype was predictive of progression. Neither hyperdiploidy nor any of the translocation subtypes was a risk factor for progression to active myeloma in S0120, which contrasts with the fluorescence in situ hybridization (FISH) findings that these factors are associated with higher rates of progression in SMM.2,3 In addition, in S0120, on univariate analysis, the GEP proliferative index and the polytypic plasma cell score were prognostic but lost significance once the powerful GEP-70 risk score was included. Since ∼30% of genes in the GEP-70 score are from chromosome 1, this part of the S0120 data is concordant with the FISH data that demonstrated that chromosome 1q21 additions are a risk factor for SMM progression.3
Based on Cox modeling, the authors generated 2 risk models for time to clinical progression requiring treatment. The first included only clinical variables: (1) serum M-spike ≥30 g/L; (2) bone marrow plasmacytosis ≥20%; and (3) age ≥65 years. The other more intriguing model included GEP and the following clinical variables: (1) GEP-70 >−0.26; (2) involved serum immunoglobulin free light chain (FLC) >250 mg/L; and (3) serum M-spike ≥30 g/L. For both systems, patients were grouped according to whether they had no risk factors, 1 risk factor, or ≥2 risk factors. The clinical model without GEP yielded 3 groups with 2-year rates to MM requiring treatment of 3%, 14%, and 40%, respectively. The GEP containing model yielded 3 groups with 2-year rates to MM of 3%, 22%, and 67%, respectively. When the 39 patients with MGUS and available GEP data were excluded and the GEP based risk model was limited to the 79 SMM patients, the respective 2-year rates to MM requiring therapy increased marginally to 3%, 29%, and 71%, respectively.
Dhodopkar et al effectively used their dataset to test other published prognostic factors and systems, such as the one developed by Dispenzieri.4 Similar risk factors to what have been previously published were found in S0120, albeit with slightly different thresholds for some of the factors. The GEP-70/serum FLC/M-spike model was stronger than other published models, but the authors neglected to test the bone marrow plasma cell >60% threshold5,6 due to a paucity of patients satisfying that criterion. The authors indirectly tested the concept that the percent of aberrant vs polytypic plasma cells are a risk for progression,7 but find that the polytypic plasma cell score, albeit using a markedly different technique, was not a significant risk if the GEP-70 score was included in the model. Greater than 1 lesion on surveillance MRI was a very strong predictor of progression,7 despite the fact that it was a very rare event in the SWOG (formerly the Southwest Oncology Group) cohort, with only 6% tested patients having >1 focal lesion in their spines.
Should all patients with MGUS and SMM have GEP-70 scores generated? Before these data can be used for more than hypothesis generation, validation will be required. Time to progression for the S0120 MGUS population tracked well with the published literature; however, the rates for time to MM requiring therapy in the SMM group did not.8-10 In the SWOG series, nearly 20% of SMM patients were deemed to have progressed at 1 year, and during the subsequent 3 years, the rate of progression was only approximately 3% per year. This contrasts markedly with the 10% per year rate of progression seen over the first 5 years seen in other series,4,9 highlighting 2 potential problems with observational studies, prospective or otherwise: (1) despite clear definitions of smoldering myeloma,11 in real-world practice, patients with newly diagnosed SMM are not infrequently siphoned off for active treatment; and (2) the decision to consider progression from SMM to MM requiring treatment can be subjective, thereby affecting estimates of time to progression.
The data from S0120 reinforce that the landscape of definitions for and treatments of plasma cell disorders is in flux.4,10 The MGUS, SMM, and MM constructs have served us well for decades, but it is clear that current definitions are imperfect (see figure). It is intuitive that a combination of clinical and genomic parameters should better define those patients with an active malignancy from those who have a premalignant condition. GEP added little prognostic information to the MGUS group, at least with the current follow-up and small sample size, with a 4-year time to progression in the presence and absence of high-risk GEP-70 was 3.5% and 0%, respectively. In contrast, the respective rates for the SMM group were 51% and 12%. S0120 alone is not sufficient to change definitions, but it brings us a step closer, especially if its findings can be validated in other MGUS and SMM cohorts. Only then might there be value for GEP to be done outside the research setting in patients with MGUS and SMM, but even then, clinical trials will be required to ascertain whether earlier intervention in patients with high-risk SMM should be treated preemptively and/or reclassified as active myeloma.4,10
Conflict-of-interest disclosure: The author declares no competing financial interests.