In this issue of Blood, Lindemans et al report on the impact of thymoglobulin (antithymocyte globulin [ATG]) during the preparative regimen for pediatric unrelated umbilical cord blood transplantation (UCBT). Survival and chronic graft-versus-host disease (GVHD) were similar whether or not patients received ATG. The patients who did not receive ATG had a lower rate of viral infections but an increased rate of acute GVHD.1
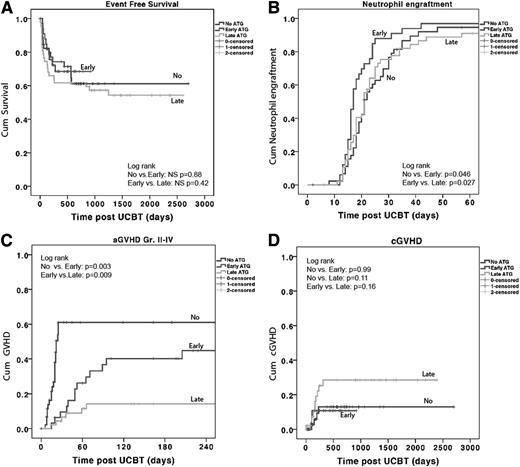
Outcomes by early, late, and no ATG groups. See Figure 1 in the article by Lindemans et al that begins on page 126.
Outcomes by early, late, and no ATG groups. See Figure 1 in the article by Lindemans et al that begins on page 126.
This year, 2013, marks the 25th anniversary of the first UCBT, performed by Gluckman et al2 in France in 1988 for a child with Fanconi anemia. More than 600 000 UCB units have been donated for public use worldwide, and more than 30 000 UCBTs have been performed. Multiple retrospective comparisons have indicated similar survival among children or adult patients receiving UCBT or a standard unrelated donor hematopoietic stem cell transplant (HSCT).3,4 The results of UCBT in children with acute leukemia and myelodysplasia have improved over time from an overall survival (OS) of 37% in 1996-1999 to 56% in 2006-2011. Likewise, adult OS has improved from 22% in 1996-1999 to 37% in 2006-2011.5 Despite these advances, infection and immune reconstitution remain significant causes of morbidity and mortality after UCBT. Are these issues with immunity related to inherent properties of the UCB product or to the use of ATG added into the conditioning regimen to prevent graft rejection?
ATG has been used as a form of in vivo T-cell depletion to reduce the risk of GVHD after allogeneic HSCT from related sibling and unrelated donors. Finke et al6 reported a randomized study of ATG-Fresenius preparation added to standard cyclosporine and methotrexate GVHD prophylaxis for patients receiving a myeloablative transplant; survival was similar in both arms, but the incidence of GVHD was lower in the ATG arm. Soiffer et al7 analyzed 1676 adults with hematologic malignancies undergoing a reduced-intensity conditioning (RIC) related or unrelated donor HSCT using fludarabine-based conditioning regimens. The relapse rate was higher and the disease-free survival lower when either ATG or alemtuzumab was added to the preparative regimen. The risk of acute GVHD was similar among patients who received either ATG or a T-cell replete HSCT. This study, however, did not include children or patients undergoing UCBT. With UCBT, several small studies have indicated a higher risk of infection with the use of ATG for UCBT, but there have been no large comparative studies to date. Delayed immune recovery after UCBT is characterized by prolonged T lymphopenia, compensatory expansion of B and natural killer cells, and late memory T-cell skewing.8 In essence, a UCBT is a naturally T-cell depleted HSCT. In a study of immune recovery after RIC HSCT, Jacobson et al9 compared 102 adult unrelated donor recipients with 42 adult double UCBT recipients, all of whom had received rabbit ATG. Reconstitution of CD3+ T cells, including naïve (CD45RO−) and memory (CD45RO+) CD4+ T cells, regulatory (CD4+CD25+) T cells, and CD8+ T cells was delayed for 6 months post-HSCT in the UCBT patients. Clinically, these findings were correlated with an increased risk of infection, including Epstein Barr virus–associated lymphoproliferative disease, in the first 6 months after UCBT.10 Lower doses of ATG may reduce this risk. Multiple successful UCBT conditioning regimens have been developed without ATG, but they have not been directly compared with ATG-containing regimens.
The article by Lindemans et al1 seeks to address this important question in a large study of 127 children receiving unrelated UCBT in London or Utrecht. Patients were divided into 3 groups: an early ATG group receiving ATG between days −9 and −5, a late ATG group receiving ATG between days −5 and 0, and a no-ATG group. The ATG administered was rabbit ATG at a total dose of 10 mg/kg. Survival was excellent overall (71% in the no-ATG group, 68% in the early ATG group, and 61% in the late ATG group), with no differences among the groups (see figure). Neutrophil recovery was also similar among the 3 groups. CD4+ T-cell counts were higher in the first year after UCBT in the patients who did not receive ATG, and this finding correlated with a lower incidence of viral reactivation and death from viral infections (P = .002) in the patients who did not receive ATG. As expected, the incidence of acute GVHD was higher in the no-ATG group, but the incidence of chronic GVHD was similar across all the groups. The authors concluded that the omission of ATG may be important to prevent viral reactivation after pediatric unrelated UCBT. They also suggest an individualized approach for each patient, based on disease status, infection history, and pharmacokinetic modeling to determine the optimal dose of ATG.
For UCBT, should the answer to ATG be yes or no? A randomized study will be required to determine whether ATG confers a survival advantage for patients undergoing UCBT. Different dosages and preparations of ATG may need to be considered, with a uniform conditioning regimen and GVHD prophylaxis. The article by Lindemans et al helps to address this ATG question, but the answer (for now) is maybe.
Conflict-of-interest disclosure: The author declares no competing financial interests.