Key Points
MK promotes PMN recruitment during the acute inflammatory response.
MK and β2 integrins (CD11/CD18) cooperate in mediating PMN adhesion during acute inflammation.
Abstract
Emerging evidence suggests a role of the cytokine midkine (MK) in inflammation. In this study, its functional relevance for recruitment of polymorphonuclear neutrophils (PMNs) during acute inflammation was investigated. Intravital microscopy and histologic analysis of tumor necrosis factor-α-stimulated cremaster muscle venules revealed severely compromised leukocyte adhesion and extravasation in MK−/− mice compared with MK+/+ animals. Systemic administration of recombinant MK completely rescued the adhesion defect in MK−/− mice. In a hind limb ischemia model, leukocyte accumulation in MK−/− mice was significantly diminished compared with MK+/+ animals. However, MK did not lead to an inflammatory activation of PMNs or endothelial cells suggesting that it does not serve as classical proinflammatory cytokine. Unexpectedly, immobilized MK mediated PMN adhesion under static and flow conditions, whereas PMN-derived MK was dispensable for the induction of adhesion. Furthermore, adhesion strengthening remained unaffected by MK. Flow cytometry revealed that immobilized, but not soluble MK, significantly promoted the high affinity conformation of β2 integrins of PMNs. Blocking studies of low-density lipoprotein receptor-related protein 1 (LRP1) suggested that LRP1 may act as a receptor for MK on PMNs. Thus, MK seems to support PMN adhesion by promoting the high affinity conformation of β2 integrins, thereby facilitating PMN trafficking during acute inflammation.
Introduction
Recruitment of polymorphonuclear neutrophils (PMNs) from the blood into the tissue, a critical prerequisite of the inflammatory response, is triggered by injury, infection, or hypoxia.1 The leukocyte recruitment cascade consists of a series of consecutive events involving the initial capturing of free-flowing leukocytes, followed by leukocyte rolling, slow rolling, arrest, adhesion strengthening, spreading, and intraluminal crawling to extravasation sites, and subsequent transendothelial diapedesis.2 Upon extravasation, PMNs can perform abluminal crawling on the pericytes lining the blood vessels until they enter the tissue for interstitial migration to sites of lesion.3 During rolling, PMNs are in intimate contact with the endothelium and can be activated by proinflammatory cytokines such as chemokine (C-X-C motif) ligand 1 (CXCL1), tumor necrosis factor-α (TNF-α), or other mediators presented on the luminal side of inflamed endothelial cells.2 Activation of PMNs leads to rapid conformational changes of the β2 integrins, lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18), or macrophage-1 antigen (Mac-1, CD11b/CD18) by inside-out signaling.4-6 The high affinity conformation of β2 integrins mediates firm arrest under flow conditions by binding to intercellular adhesion molecule 1 (ICAM1) on inflamed endothelial cells allowing subsequent extravasation and migration to sites of lesion.7,8
Emerging evidence has shown that midkine (MK), the 13 kilodalton founding member of a family of so-called heparin-binding growth factors, promotes inflammation.9-13 The human MK gene is induced during hypoxic or inflammatory conditions due to the presence of hypoxia and nuclear factor κB-responsive elements upstream of its promoter sequence.14,15 MK is expressed in PMNs, monocytes, and lymphocytes, and released by endothelial cells under hypoxic conditions.16-18 MK binds to various receptors with high affinity, including heparan and chondroitin sulfate proteoglycans,19,20 β1 integrins,21 and the low-density lipoprotein receptor-related protein 1 (LRP1).22 Interestingly, LRP1 interacts with β2 integrins, thereby promoting leukocyte adhesion.23-25
MK has been implicated in a wide number of biological functions including for example, angiogenesis under hypoxic conditions,18 cell survival,26 and tumor growth.27 Multiple studies also suggest an important role for MK in several pathological inflammatory conditions.10,11 Accordingly, MK is significantly upregulated in inflamed synovial tissue during an active rheumatoid arthritis flare in contrast to synovial tissue of healthy patients.9 This is further confirmed by findings in a mouse arthritis model, where leukocyte infiltration and joint damage are markedly attenuated in MK−/− mice.10 Similar results were obtained in inflammatory animal models of inflammatory bowel disease28 and multiple sclerosis, as well.11
Thus, a growing body of evidence proposes a functional role of MK for leukocyte extravasation and tissue infiltration during inflammation; however, it is unclear how and whether MK is directly involved in the leukocyte recruitment cascade. In this study, we show that MK supports adhesion of PMNs by promoting the high affinity conformation of β2 integrins, and thus contributes to the control of PMN trafficking during inflammation.
Material and methods
Mice
MK−/− mice (Mdktm1Tmn) were maintained on the C57BL/6J genetic background.29 CD18−/− mice (Itgb2tm1Bay)30 were generously provided by Karin Scharffetter-Kochanek (Department of Dermatology and Allergic Diseases, University of Ulm, Ulm, Germany) and used on a mixed 129Sv × C57BL/6 or a C57BL/6 genetic background. CD29flox/flox mice (Itgb1tm1Ref)31 on a C57BL/6 genetic background were a kind gift from Reinhard Fässler (Department of Molecular Medicine, Max-Planck-Institute of Biochemistry, Martinsried, Germany). Animal experiments were institutionally approved by Regierung von Oberbayern (Munich, Germany).
Reagents and antibodies
Bovine serum albumin, casein, crystal violet, human and murine fibrinogen (FG), glutaraldehyde, glycine, glucose, Percoll, recombinant human (rh) TNF-α, and sodium chloride were obtained from Sigma (Deisenhofen, Germany). Fetal calf serum, Hanks balanced salt solution, penicillin/streptomycin, poly-L-lysine, phosphate-buffered saline, RPMI 1640 (medium), and trypsin were purchased from Biochrom AG (Berlin, Germany). Ethylenediaminetetraacetic acid, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and trypan blue were ordered from AppliChem (Darmstadt, Germany). Collagenase was delivered by Roche (Mannheim, Germany) and endothelial cell growth medium by PromoCell (Heidelberg, Germany). rh MK was purchased from PeproTech (Rocky Hill, NJ); recombinant murine TNF-α and rh and rmICAM1 were purchased from R&D Systems (Minneapolis, MN); and rmCXCL1 was purchased from BioSource (Nivelles, Belgium). The phycoerythrin (PE)-conjugated monoclonal rat anti-mouse CD18 (clone C71/16), CD11a (clone 2D7), GR-1 (clone RB6-8C5) antibodies, and the PE-conjugated rat IgG2a (clone R35-95) control antibody, as well as the function-blocking monoclonal rat anti-mouse CD18 (clone GAME-46) and the unconjugated monoclonal rat anti-mouse CD45 antibody (clone 30-F11) were obtained from BD Biosciences (Heidelberg, Germany). The PE-conjugated monoclonal rat anti-mouse CD11b (clone M1/70.15) and the PE-conjugated rat IgG2b control antibody were ordered from ImmunoTools GmbH (Friesoythe, Germany). The PE-conjugated monoclonal hamster anti-mouse CD29 antibody (clone HMs1-1) and the PE-conjugated hamster IgG isotype control (clone HTK888) were ordered from BioLegend (San Diego, CA). The fluorescein isothiocyanate (FITC)-conjugated monoclonal mouse anti-human ICAM1 (clone 6.5B5) was received from DAKO (Glostrup, Denmark). The FITC-conjugated monoclonal mouse anti-human vascular cell adhesion molecule 1 (clone 1.G11B1) and the FITC-conjugated mouse IgG1 control antibody (clone 15H6) were purchased from SouthernBiotech (Birmingham, AL). The monoclonal function-blocking rat anti-mouse CD49d antibody (clone PS/2) was delivered from AbD Serotec (Kidlington, UK). The monoclonal mouse antibody recognizing the high affinity conformation of CD11/18 (clone mAb24) was obtained from Hycult Biotech (Plymouth Meeting, PA). The unconjugated rat IgG2b isotype control antibody was purchased from R&D Systems. The secondary Alexa Fluor 488 conjugated F(ab′)2 fragment of goat anti-mouse IgG (clone A11017) was ordered from Invitrogen (Carlsbad, CA). Human blood collection was conducted according to the Declaration of Helsinki and approved by the Institutional Ethics Committee of Ludwig-Maximilians-University.
Intravital microscopy
Preparation and analysis of cremaster muscle whole mounts
To assess leukocyte extravasation, cremaster muscles were surgically prepared 2 hours after intrascrotal application of TNF-α (500 ng) as reported.34 For further details, see supplemental Methods.
Flow cytometry
For analysis of Gr-1 or β2 integrin expression, murine PMNs (2 × 105/sample) were left untreated or stimulated with soluble MK (100 ng/mL) or TNF-α (100 ng/mL) for 20 minutes at 37°C and stained with the PE-conjugated rat anti-mouse Gr-1, CD11a, CD11b, CD18, IgG2a, or IgG2b isotype control antibodies as previously described.35 To study MK binding to CD18−/−, CD29−/−, or wild-type (WT) PMNs, cells (105/sample) were left untreated or stimulated with TNF-α (100 ng/mL), CXCL1 (100 ng/mL), or MnCl2 (3 mM) and subsequently incubated with Alexa Fluor 488-labeled MK (30 μg/mL) for 20 minutes at 37°C. When indicated, LRP-associated protein (LRPAP [3 μM]), the anti-CD49d (20 μg/mL), or the IgG2b control antibody (20 μg/mL) were used as blocking agents for 20 minutes at room temperature (RT) prior to stimulation. The high affinity conformation of β2 integrins on human PMNs (1.25 × 105/sample) was analyzed using the mAb24 antibody36 and the secondary Alexa Fluor 488-conjugated F(ab′)2 fragment in the presence or absence of microparticles. Cells were subsequently left untreated, or stimulated with soluble MK (100 ng/mL, 30 μg/mL), or TNF-α (100 ng/mL) for 15 minutes at 37°C. Cells bound to microparticles vs unbound cells were identified by a shift in the forward and side scatter plot. Unspecific binding was analyzed using casein-coated microparticles and subtracted from the obtained values. All experiments were conducted using FACSCanto II flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Hind limb ischemia and immunohistochemical analyses
Isolation of human PMNs
Isolation of human PMNs using a discontinuous isotonic Percoll gradient was performed as previously described.38 For further details, see supplemental Methods.
Isolation of murine bone marrow PMNs
Adhesion of PMNs under static conditions
Murine or human PMNs (105/well) were suspended in adhesion medium and plated onto 96-well microtiter plates coated with murine (50 µg/mL) or human (250 µg/mL) FG, murine ICAM1 (5 µg/mL), or human MK (2 µg/mL) as described previously.41 For further details, see supplemental Methods.
Adhesion and adhesion strengthening of PMNs under flow conditions
PMN adhesion and adhesion strengthening were analyzed in vitro using IBIDI μ-Slide VI 0.1 flow chambers (IBIDI, Martinsried, Germany) as previously described.5 For further details, see supplemental Methods.
Protein coating of microparticles
Carboxylated polystyrene microparticles (3 μm; cat #09850) were obtained from Polysciences, Inc. (Warrington, PA). MK, ICAM1, and casein (100 μg/mL) were covalently coupled to the microparticles using the “Carbodiimide” protocol provided by the manufacturer. Unreacted sites on the microparticles were blocked with glycine (0.25 M) and casein (0.25 M).
Alexa Fluor 488 labeling of MK
Recombinant MK (rMK) was fluorescently labeled with Alexa Fluor 488 Microscale Protein Labeling Kit (cat #A30006, Molecular Probes, Paisley, UK) according to the manufacturer’s protocol.
Statistical analysis
Data shown represents mean or median ± standard error of the mean (SEM). Statistical significance (P < .05) was determined with SigmaPlot v10.0 (Systat Software, Chicago, IL) using Student's t test, Mann-Whitney rank sum test, or Kruskal-Wallis analysis of variance test, respectively.
Results
MK was critical for leukocyte adhesion and extravasation in vivo
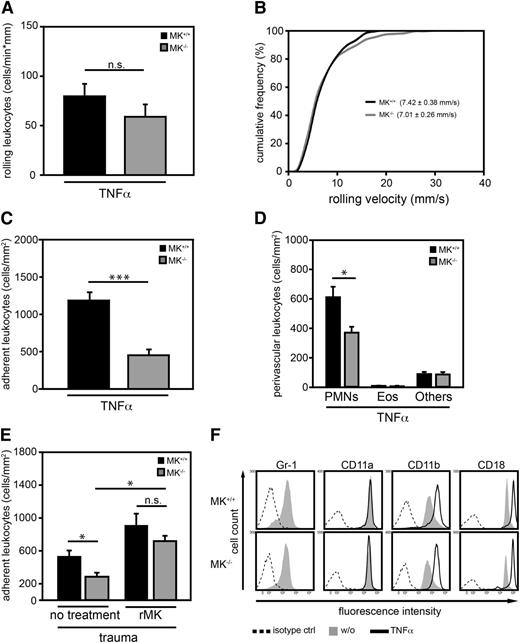
To analyze the relevance of MK for leukocyte recruitment to the site of inflammation, intravital microscopy of cremaster muscle venules of MK+/+ or MK−/− mice was conducted 2 hours after intrascrotal injection of TNF-α. White blood cell counts, hemodynamic, and microvascular parameters of mice (supplemental Table 1), as well as the number and velocity of rolling leukocytes in postcapillary venules were similar between the two mouse strains (Figure 1A-B). In contrast, leukocyte adhesion was significantly compromised in MK−/− mice compared with control animals suggesting that endogenous MK was critical for leukocyte adhesion (Figure 1C). Histologic analysis of cremaster muscle whole mounts revealed that only extravasation of PMNs (but not eosinophils or other leukocytes) was significantly diminished in MK−/− mice compared with MK+/+ animals, suggesting that MK specifically impacted PMN extravasation (Figure 1D). To investigate the role of MK in sterile inflammation, the trauma model was performed in which leukocyte recruitment is induced only through surgical preparation of the cremaster muscle. Intravital microscopy of postcapillary venules in the cremaster muscle of MK+/+ and MK−/− mice was performed directly after exteriorization of the cremaster muscle. In the trauma model, white blood cell counts, hemodynamic, and microvascular parameters of MK+/+ and MK−/− mice before and after application of rMK were comparable (supplemental Table 2). In sharp contrast, leukocyte adhesion in MK−/− mice was significantly diminished compared with MK+/+ animals (Figure 1E). Systemic application of 1 μg rMK significantly induced adhesion, and thereby rescued the phenotype of MK−/− mice with no significant difference between the mouse strains concerning the number of adherent leukocytes. Together, these data suggest that endogenous MK is crucial for mediating adhesion and subsequent extravasation of PMNs during acute inflammation in vivo. Next, we investigated whether compromised maturation of PMNs or altered expression of β2 integrins, which are critical for PMN adhesion,42 were responsible for the observed adhesion and extravasation defect in MK−/− mice in vivo. The expression of Gr-1, a maturation marker of PMNs, and the β2 integrin subunits CD11a, CD11b, or CD18 were examined in PMNs derived from MK+/+ or MK−/− mice (Figure 1F). Flow cytometric analysis revealed that expression of all surface molecules tested were similar between the two groups indicating that the observed adhesion defect in MK−/− mice was not due to compromised maturation or altered β2 integrin expression of PMNs.
MK was critical for leukocyte adhesion and extravasation in inflamed cremaster muscle venules. (A-C) Intravital microscopy of cremaster muscle postcapillary venules in MK+/+ and MK−/− mice 2 hours after intrascrotal administration of TNF-α (500 ng). (A) Number of rolling leukocytes; n = 21 venules from 5 MK+/+ mice, n = 24 venules from 5 MK−/− mice. (B) Leukocyte rolling velocity was analyzed offline; accumulated frequency of rolling velocity includes 224 cells from 5 MK+/+ mice and 221 cells from 5 MK−/− mice. (C) Number of adherent leukocytes as analyzed by intravital microscopy; n = 21 venules from 5 MK+/+ mice, n = 24 venules from 5 MK−/− mice. (D) Differential cell counts of perivascular leukocytes as assessed in cremaster muscle whole mounts were determined morphologically. (E) Analysis of leukocyte adhesion in cremaster muscle venules of MK+/+ or MK−/− mice during trauma-induced inflammation before (no treatment) and 10 minutes after systemic application of rMK using intravital microscopy. For MK+/+ mice, no treatment: n = 24 venules from 6 mice; rMK: n = 9 venules from 3 mice; for MK−/− mice, no treatment: n = 21 venules from 6 mice; rMK: n = 10 venules from 3 mice. (F) Flow cytometric analysis of cell surface expression of Gr-1, as well as expression of CD11a, CD11b, or CD18 in unstimulated (w/o) or TNF-α (100 ng/ml) treated PMNs from MK+/+ or MK−/− mice following 20 minutes incubation at 37°C. Histograms are representative of 3 independent experiments. (A-E) Show mean ± SEM. *P < .05; ***P < .001. Eos, eosinophils; Others, lymphocytes and basophils; n.s., not significant.
MK was critical for leukocyte adhesion and extravasation in inflamed cremaster muscle venules. (A-C) Intravital microscopy of cremaster muscle postcapillary venules in MK+/+ and MK−/− mice 2 hours after intrascrotal administration of TNF-α (500 ng). (A) Number of rolling leukocytes; n = 21 venules from 5 MK+/+ mice, n = 24 venules from 5 MK−/− mice. (B) Leukocyte rolling velocity was analyzed offline; accumulated frequency of rolling velocity includes 224 cells from 5 MK+/+ mice and 221 cells from 5 MK−/− mice. (C) Number of adherent leukocytes as analyzed by intravital microscopy; n = 21 venules from 5 MK+/+ mice, n = 24 venules from 5 MK−/− mice. (D) Differential cell counts of perivascular leukocytes as assessed in cremaster muscle whole mounts were determined morphologically. (E) Analysis of leukocyte adhesion in cremaster muscle venules of MK+/+ or MK−/− mice during trauma-induced inflammation before (no treatment) and 10 minutes after systemic application of rMK using intravital microscopy. For MK+/+ mice, no treatment: n = 24 venules from 6 mice; rMK: n = 9 venules from 3 mice; for MK−/− mice, no treatment: n = 21 venules from 6 mice; rMK: n = 10 venules from 3 mice. (F) Flow cytometric analysis of cell surface expression of Gr-1, as well as expression of CD11a, CD11b, or CD18 in unstimulated (w/o) or TNF-α (100 ng/ml) treated PMNs from MK+/+ or MK−/− mice following 20 minutes incubation at 37°C. Histograms are representative of 3 independent experiments. (A-E) Show mean ± SEM. *P < .05; ***P < .001. Eos, eosinophils; Others, lymphocytes and basophils; n.s., not significant.
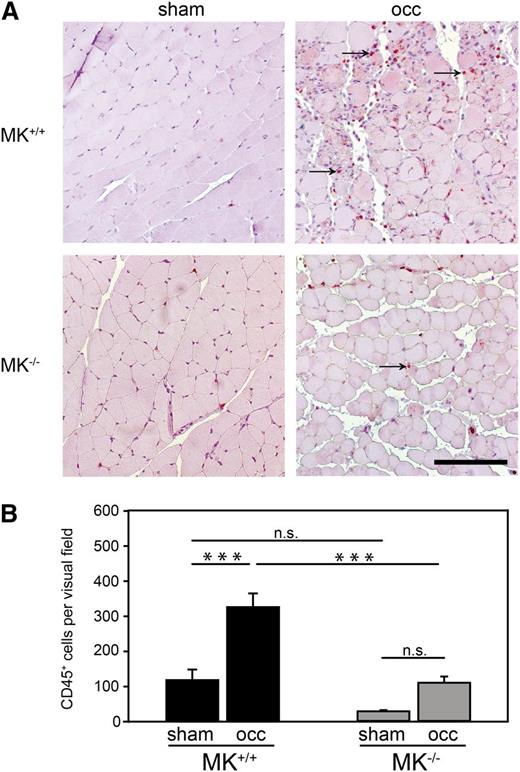
To study the role of MK in a clinically relevant setting of inflammation, we used the hind limb ischemia model analyzing leukocyte extravasation in MK+/+ and MK−/− mice. For induction of ischemia in the M. gastrocnemius, the femoral artery of the right leg of each animal was ligated for 4 days (occ), while the left leg was sham operated (sham) as control. Histologic analysis of muscle cross sections was performed by staining cells with the leukocyte marker CD45 (Figure 2A). As expected, in MK+/+ mice, the number of extravasated CD45+ cells was significantly increased in the ischemic muscle compared with the sham operation. In contrast, no significant increase of leukocyte extravasation upon induction of ischemia was observed in the genetic absence of MK. Quantitative analysis showed that leukocyte infiltration into the ischemic muscle of MK−/− was significantly reduced in comparison with MK+/+ mice (Figure 2B), supporting the concept that MK promoted leukocyte extravasation during an inflammatory response. However, MK did not induce inflammatory activation of PMNs or endothelial cells suggesting that it does not serve as a classical proinflammatory cytokine (for details, please see supplemental Figure 1A-C).
Leukocyte extravasation was compromised in MK−/−mice during hypoxia-mediated inflammation. (A) Representative histological sections of Mm. gastrocnemii from MK+/+ or MK−/− mice 4 days after ligation of the femoral artery in the right leg (occ) and sham operation of the left leg (sham). Immunohistochemical staining of CD45+ cells (red) indicated by arrows. Bar = 100 μm. (B) Quantitative analysis of leukocyte extravasation measured as CD45+ cells per visual field; n = 3 independent experiments. Diagram shows mean ± SEM. ***P < .001.
Leukocyte extravasation was compromised in MK−/−mice during hypoxia-mediated inflammation. (A) Representative histological sections of Mm. gastrocnemii from MK+/+ or MK−/− mice 4 days after ligation of the femoral artery in the right leg (occ) and sham operation of the left leg (sham). Immunohistochemical staining of CD45+ cells (red) indicated by arrows. Bar = 100 μm. (B) Quantitative analysis of leukocyte extravasation measured as CD45+ cells per visual field; n = 3 independent experiments. Diagram shows mean ± SEM. ***P < .001.
Immobilized MK substantially mediated adhesion via a CD18-dependent mechanism in vitro
To study whether MK can directly mediate adhesion, a static adhesion assay was conducted. Isolated human or murine WT PMNs were left untreated for control (w/o), or stimulated with soluble MK (10, 30, 100 ng/mL), or TNF-α (100 ng/mL) upon exposure to immobilized FG. As expected, TNF-α markedly induced adhesion of human or murine PMNs compared with the unstimulated control (Figure 3A). However, stimulation with soluble MK at concentrations up to 100 ng/mL had no effect on adhesion of human PMNs and showed only a minimal effect on adhesion of murine PMNs. In contrast, immobilized MK as adhesive substratum substantially induced adhesion of human or murine PMNs without further stimulation compared with adhesion on immobilized FG (Figure 3B).
Adhesion mediated by immobilized MK in vitro was CD18 dependent. (A-D) To measure adhesion of PMNs, cells were left untreated for control (w/o) or stimulated for 10 minutes at 37°C. Adhesion is expressed as a percentage of all cells added to poly-L-lysine (100%). (A) Adhesion of human or murine WT PMNs on immobilized FG left untreated (w/o) for control, or stimulated with soluble MK (10 ng/ml, 30 ng/ml, or 100 ng/ml), or TNF-α (100 ng/ml) (n = 18 [human]; n = 3 [murine]). (B) Adhesion of human or murine WT PMNs on immobilized FG or immobilized MK either left untreated (w/o) or stimulated with TNF-α (100 ng/ml) (n = 4 [human]; n = 4 [murine]). (C) Adhesion of murine WT PMNs on immobilized ICAM1 or MK. Cells were left untreated or preincubated with a CD18 function-blocking antibody (anti-CD18) for 20 minutes at RT. Subsequently, cells were left unstimulated (w/o) for control or stimulated with TNF-α (100 ng/ml) (n = 6 [ICAM1]; n = 9 [MK]). (D) Isolated CD18+/+ or CD18−/− murine PMNs on immobilized ICAM1 or MK. Diagrams show percentage of adhesion without stimulation (w/o) or after stimulation with TNF-α (100 ng/ml) (n = 3). (E) Induction of adhesion of murine WT PMNs under flow conditions (1 dyne/cm2). Microflow chambers were coated with P-selectin (10 µg/ml) and ICAM1 (12.5 µg/ml) alone (P-selectin + ICAM1), or combined with MK (P-selectin + ICAM1 + MK: 10 µg/ml). Data show the total number of adherent PMNs at indicated times (n = 5). (F) Adhesion of isolated MK+/+ and MK−/− PMNs under flow conditions. Microflow chambers were coated with P-selectin (10 µg/ml), ICAM1 (12.5 µg/mL), and CXCL1 (5 µg/ml) (n = 4). (G) Adhesion strengthening of adherent murine WT PMNs under gradually increasing shear stress (0.2-8.0 dyne/cm2). WT PMNs were seeded into microflow chambers coated with ICAM1 (ICAM1: 12.5 µg/ml) alone, or in combination with MK (ICAM1 + MK: 10 µg/ml), or CXCL1 (ICAM1 + CXCL1: 5 µg/ml) for 10 minutes before flow was applied as indicated. Adhesion strengthening was measured as the number of adherent PMNs in percent of initially adherent cells at 0.2 dyne/cm2 (100%) (n = 5). (A-E) Show mean ± SEM. *P < .05; ***P < .001.
Adhesion mediated by immobilized MK in vitro was CD18 dependent. (A-D) To measure adhesion of PMNs, cells were left untreated for control (w/o) or stimulated for 10 minutes at 37°C. Adhesion is expressed as a percentage of all cells added to poly-L-lysine (100%). (A) Adhesion of human or murine WT PMNs on immobilized FG left untreated (w/o) for control, or stimulated with soluble MK (10 ng/ml, 30 ng/ml, or 100 ng/ml), or TNF-α (100 ng/ml) (n = 18 [human]; n = 3 [murine]). (B) Adhesion of human or murine WT PMNs on immobilized FG or immobilized MK either left untreated (w/o) or stimulated with TNF-α (100 ng/ml) (n = 4 [human]; n = 4 [murine]). (C) Adhesion of murine WT PMNs on immobilized ICAM1 or MK. Cells were left untreated or preincubated with a CD18 function-blocking antibody (anti-CD18) for 20 minutes at RT. Subsequently, cells were left unstimulated (w/o) for control or stimulated with TNF-α (100 ng/ml) (n = 6 [ICAM1]; n = 9 [MK]). (D) Isolated CD18+/+ or CD18−/− murine PMNs on immobilized ICAM1 or MK. Diagrams show percentage of adhesion without stimulation (w/o) or after stimulation with TNF-α (100 ng/ml) (n = 3). (E) Induction of adhesion of murine WT PMNs under flow conditions (1 dyne/cm2). Microflow chambers were coated with P-selectin (10 µg/ml) and ICAM1 (12.5 µg/ml) alone (P-selectin + ICAM1), or combined with MK (P-selectin + ICAM1 + MK: 10 µg/ml). Data show the total number of adherent PMNs at indicated times (n = 5). (F) Adhesion of isolated MK+/+ and MK−/− PMNs under flow conditions. Microflow chambers were coated with P-selectin (10 µg/ml), ICAM1 (12.5 µg/mL), and CXCL1 (5 µg/ml) (n = 4). (G) Adhesion strengthening of adherent murine WT PMNs under gradually increasing shear stress (0.2-8.0 dyne/cm2). WT PMNs were seeded into microflow chambers coated with ICAM1 (ICAM1: 12.5 µg/ml) alone, or in combination with MK (ICAM1 + MK: 10 µg/ml), or CXCL1 (ICAM1 + CXCL1: 5 µg/ml) for 10 minutes before flow was applied as indicated. Adhesion strengthening was measured as the number of adherent PMNs in percent of initially adherent cells at 0.2 dyne/cm2 (100%) (n = 5). (A-E) Show mean ± SEM. *P < .05; ***P < .001.
To investigate the relevance of β2 integrins for PMN adhesion in the presence of immobilized MK, an adhesion assay was performed on immobilized ICAM1, or immobilized MK without further stimulation (w/o), or after stimulation with TNF-α (100 ng/mL). The addition of a function-blocking anti-CD18 antibody significantly reduced adhesion on ICAM1 compared with untreated controls (untreated). Similar effects were observed on immobilized MK (Figure 3C). These results were confirmed using PMNs from CD18+/+ or CD18−/− mice (Figure 3D), suggesting that CD18 is critically required for PMN adhesion in the presence of immobilized MK.
To further define the role of MK for PMN adhesion under physiological flow conditions, we performed an adhesion assay using microflow chambers coated with P-selectin and ICAM1 alone, or combined with MK (Figure 3E). Here, MK together with P-selectin and ICAM1 was able to significantly increase adhesion of PMNs compared with P-selectin and ICAM1 alone. These results indicate that MK promotes PMN adhesion under physiological flow conditions in vitro. To study the impact of PMN-derived MK for the induction of adhesion, we performed adhesion experiments in microflow chambers coated with immobilized P-selectin, ICAM1, and CXCL1 using isolated MK+/+ and MK−/− PMNs (Figure 3F). However, adhesion was unaffected in the genetic absence of MK, suggesting that PMN-derived MK was dispensable for the induction of adhesion.
In order to determine whether MK can induce adhesion strengthening, detachment assays using microflow chambers coated with ICAM1, ICAM1 and MK, or ICAM1 and CXCL1 as positive control were conducted (Figure 3G). As expected, adhesion strengthening was markedly induced by CXCL1 compared with ICAM1 alone. However, immobilized MK did not affect adhesion strengthening on ICAM1. These findings demonstrate that MK specifically promotes the induction of adhesion but seems to have no impact on adhesion strengthening after initial arrest.
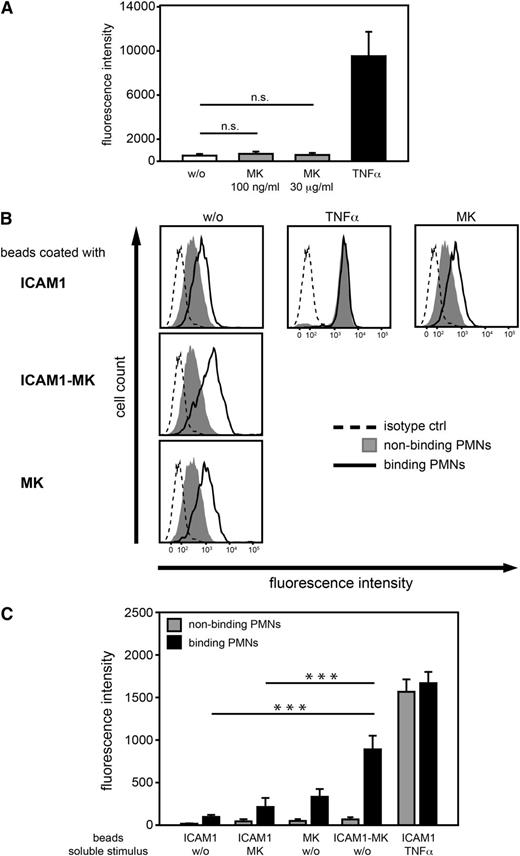
Immobilized MK promoted the high affinity conformation of β2 integrins
To analyze the mechanism by which MK induced adhesion via β2 integrins, binding of the mAb24 antibody, which recognizes the high affinity conformation of the I-domain of CD1836 was analyzed by flow cytometry. Isolated human PMNs were left untreated for control (w/o) or stimulated with soluble MK at low (100 ng/mL), or high concentrations (30 µg/mL), or with TNF-α (100 ng/mL). Stimulation with soluble MK at either concentration did not lead to a significant increase of mAb24 binding, suggesting that soluble MK did not have any effect on the high affinity conformation of β2 integrins (Figure 4A). In order to study the impact of immobilized MK on the high affinity form, microparticles (beads) coated with ICAM1, ICAM1 and MK (ICAM1-MK), or MK alone were incubated with human PMNs. In addition, cells were left untreated for control or either stimulated with TNF-α (100 ng/mL) or soluble MK (100 ng/mL). Within each sample, mAb24 binding of the cell populations that had bound (binding PMNs) or had not bound (nonbinding PMNs) microparticles, was determined (Figure 4B). Increased mAb24 epitope expression of the nonbinding population was only observed after stimulation with TNF-α, whereas soluble MK had no substantial effect compared with the unstimulated control. In PMNs that had bound to ICAM1-MK-coated microparticles, mAb24 binding was profoundly increased compared with PMNs that had bound only ICAM1 coated microparticles without further treatment (w/o). In addition, the presence of immobilized MK in the absence of ICAM1 had only minor effects. These findings were confirmed by quantitative analysis of mAb24 binding (Figure 4C). Thus, these results suggest that immobilized MK, together with ICAM1, promoted PMN adhesion via the high affinity conformation of β2 integrins.
The high affinity conformation of β2integrins was promoted by immobilized MK. (A-C) Flow cytometric analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody. (A) Quantitative analysis of median fluorescence intensity of isolated PMNs left unstimulated (w/o) or stimulated with soluble MK at various concentrations (100 ng/ml or 30 μg/ml), or TNF-α (100 ng/ml) for 15 minutes at 37°C (n = 4). (B) Analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody or control antibody following incubation with microparticles coated with ICAM1 (ICAM1), ICAM1 and MK (ICAM1-MK), or MK (MK), and left untreated (w/o) or after stimulation with soluble MK (100 ng/ml), or TNF-α (100 ng/ml) for 15 minutes at 37°C. Histograms are representative of 6 independent experiments. (C) Quantitative analysis of median fluorescence intensity of mAb24 epitope expression of PMNs binding or nonbinding to coated microparticles (n = 6). (A, C) Show median ± SEM. ***P < .001.
The high affinity conformation of β2integrins was promoted by immobilized MK. (A-C) Flow cytometric analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody. (A) Quantitative analysis of median fluorescence intensity of isolated PMNs left unstimulated (w/o) or stimulated with soluble MK at various concentrations (100 ng/ml or 30 μg/ml), or TNF-α (100 ng/ml) for 15 minutes at 37°C (n = 4). (B) Analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody or control antibody following incubation with microparticles coated with ICAM1 (ICAM1), ICAM1 and MK (ICAM1-MK), or MK (MK), and left untreated (w/o) or after stimulation with soluble MK (100 ng/ml), or TNF-α (100 ng/ml) for 15 minutes at 37°C. Histograms are representative of 6 independent experiments. (C) Quantitative analysis of median fluorescence intensity of mAb24 epitope expression of PMNs binding or nonbinding to coated microparticles (n = 6). (A, C) Show median ± SEM. ***P < .001.
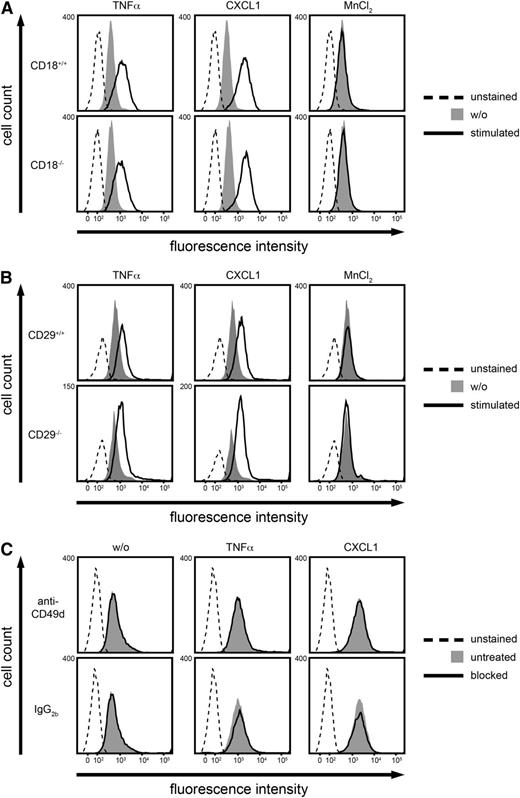
MK binding to PMNs did not require β2 or β1 integrins
To study which receptor was involved in the binding of MK to PMNs, Alexa Fluor 488-labeled MK (30 µg/mL) was incubated with murine PMNs, and MK binding was subsequently analyzed using flow cytometry. PMNs were left untreated for control (w/o) or stimulated with TNF-α (100 ng/mL), CXCL1 (100 ng/mL), or MnCl2 (3 mM) (Figure 5A-B). Interestingly, stimulation with TNF-α or CXCL1 increased MK binding to PMNs compared with the unstimulated control, whereas MnCl2 did not influence MK binding. In order to study whether β2 or β1 integrins were involved, MK binding to isolated PMNs from CD18−/− or CD29−/− mice was analyzed (Figure 5A-B). However, no difference was observed. To further confirm that MK did not bind to α4β1 integrins, CD49d (the α subunit of the β1 integrin α4β1) was blocked with a function-blocking antibody, again showing no significant difference in MK binding compared with the untreated control (Figure 5C). These findings suggest that MK binding to PMNs did not depend on β2 or β1 integrins.
MK binding to PMNs did not require CD18 or CD29. (A-C) Analysis of binding of Alexa Fluor 488-labeled MK to murine PMNs using flow cytometry. For control, PMNs were left unstained. (A-B) Cells were left untreated for control (w/o) or stimulated with TNF-α (100 ng/ml), CXCL1 (100 ng/ml), or MnCl2 (3 mM) for 20 minutes at 37°C. (A) Binding of MK to isolated PMNs from CD18+/+ or CD18−/− mice. (B) Binding of MK to isolated PMNs from CD29+/+ or CD29−/− mice. (C) Binding of MK to PMNs from WT mice. The α4 subunit was blocked with the anti-CD49d antibody or left unblocked (untreated), and the rat IgG2b antibody was used as control. Blocking was performed for 20 minutes at RT and cells were subsequently left unstimulated (w/o) or stimulated with TNF-α (100 ng/ml), or CXCL1 (100 ng/ml) for 20 minutes at 37°C. Histograms (A-C) are representative of 3 independent experiments.
MK binding to PMNs did not require CD18 or CD29. (A-C) Analysis of binding of Alexa Fluor 488-labeled MK to murine PMNs using flow cytometry. For control, PMNs were left unstained. (A-B) Cells were left untreated for control (w/o) or stimulated with TNF-α (100 ng/ml), CXCL1 (100 ng/ml), or MnCl2 (3 mM) for 20 minutes at 37°C. (A) Binding of MK to isolated PMNs from CD18+/+ or CD18−/− mice. (B) Binding of MK to isolated PMNs from CD29+/+ or CD29−/− mice. (C) Binding of MK to PMNs from WT mice. The α4 subunit was blocked with the anti-CD49d antibody or left unblocked (untreated), and the rat IgG2b antibody was used as control. Blocking was performed for 20 minutes at RT and cells were subsequently left unstimulated (w/o) or stimulated with TNF-α (100 ng/ml), or CXCL1 (100 ng/ml) for 20 minutes at 37°C. Histograms (A-C) are representative of 3 independent experiments.
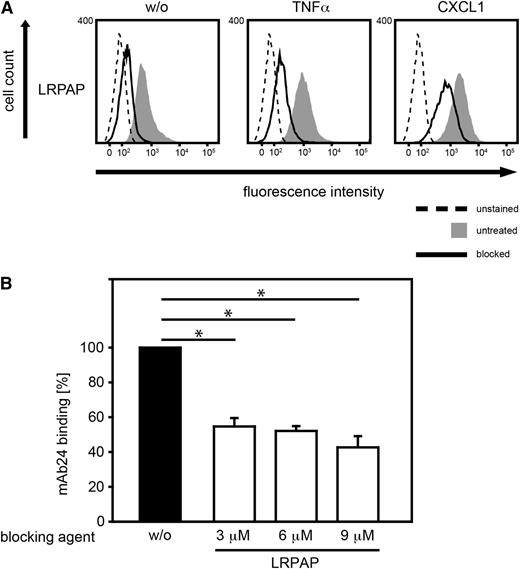
Blocking of LRP1 impaired MK binding to PMNs
Next, we studied MK binding in the presence of LRPAP (3 µM), which functionally blocks LRP1.43 We found that LRPAP significantly reduced MK binding to murine PMNs suggesting that LRP1 was involved in binding of MK (Figure 6A). To investigate whether LRP1 can act as a functional receptor for MK, promoting the high affinity conformation of β2 integrins, PMNs were incubated with LRPAP (3, 6, 9 µM) for 20 minutes at RT or left untreated for control (w/o) before binding to ICAM1 and MK-coated beads was allowed (Figure 6B). Interestingly, blockade of LRP1 significantly reduced mAb24 binding to human PMNs in the presence of ICAM1-MK coated microparticles suggesting that the effect of MK on the high affinity conformation of β2 integrins may involve LRP1. Thus, MK and β2 integrins seem to cooperate in mediating PMN adhesion via LRP1 during PMN recruitment in acute inflammation.
Blocking of LRP1-impaired MK binding to PMNs. (A) Binding of Alexa Fluor 488-labeled MK to WT PMNs using flow cytometry. For control, PMNs were left unstained. PMNs were treated with LRPAP (3 µM, blocked) for 20 minutes at RT or left untreated, and cells were subsequently left unstimulated (w/o) or stimulated with TNF-α (100 ng/ml), or CXCL1 (100 ng/ml) for 20 minutes at 37°C. Histograms are representative of 3 independent experiments. (B) Analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody following incubation with ICAM1 and MK-coated microparticles for 15 minutes at 37°C. Prior to exposure to microparticles, PMNs were treated with LRPAP (3, 6, 9 µM) or left untreated for control (w/o). Data represents percentage of median fluorescence intensity of PMNs interacting with beads after LRP1 blockade in comparison with unblocked samples (n = 5). (B) Shows median ± SEM. *P < .05.
Blocking of LRP1-impaired MK binding to PMNs. (A) Binding of Alexa Fluor 488-labeled MK to WT PMNs using flow cytometry. For control, PMNs were left unstained. PMNs were treated with LRPAP (3 µM, blocked) for 20 minutes at RT or left untreated, and cells were subsequently left unstimulated (w/o) or stimulated with TNF-α (100 ng/ml), or CXCL1 (100 ng/ml) for 20 minutes at 37°C. Histograms are representative of 3 independent experiments. (B) Analysis of the high affinity conformation of β2 integrins of human PMNs using the mAb24 antibody following incubation with ICAM1 and MK-coated microparticles for 15 minutes at 37°C. Prior to exposure to microparticles, PMNs were treated with LRPAP (3, 6, 9 µM) or left untreated for control (w/o). Data represents percentage of median fluorescence intensity of PMNs interacting with beads after LRP1 blockade in comparison with unblocked samples (n = 5). (B) Shows median ± SEM. *P < .05.
Discussion
In this study, we demonstrated that MK was critically involved in the recruitment of PMNs during acute inflammation, playing a key role for adhesion, and subsequent extravasation. Analysis of inflamed postcapillary cremaster muscle venules using intravital microscopy revealed that leukocyte adhesion was diminished upon TNF-α stimulation in the genetic absence of MK, suggesting a pivotal role of MK by promoting leukocyte adhesion in vivo. In contrast, the number of rolling leukocytes, as well as the rolling velocity was unaffected when MK was absent. Reduced leukocyte adhesion was also obtained in the trauma model of MK−/− mice. Here, administration of MK restored the adhesion defect in MK−/− animals, further supporting the concept that MK may represent a critical player in the leukocyte recruitment cascade. Via its heparin binding sites, MK is able to bind to heparan sulfates, which represent the most abundant proteoglycans of the glycocalyx of endothelial cells. Thus, MK released from endothelial cells can bind to the luminal site of endothelial cells.44 Accordingly, MK administered to MK−/− mice by intra-arterial injection may have rescued the observed adhesion defect by binding heparan sulfates on the endothelial surface. Whole mount histology data indicated that only extravasation of PMNs but not other leukocytes were affected, suggesting a specific role of MK for PMN trafficking in the experimental setting used. However, it cannot be excluded that MK has an impact on the recruitment of other inflammatory cells in different models. In line with the results obtained in the cremaster model, leukocyte extravasation was observed to be markedly compromised in the hind limb ischemia model, suggesting an essential role of MK for leukocyte recruitment in the context of hypoxia-induced inflammation as well. Due to staining with the pan leukocyte marker CD45, this experiment does not allow us to distinguish between the different leukocyte subsets. Flow cytometric analysis of Gr-1 and β2 integrin expression levels was performed to exclude the possibility that PMNs from MK−/− mice had defects in maturation or β2 integrin expression.
The observed in vivo data are in line with findings from inflammation models using MK−/− animals, where the pathological outcome was ameliorated and leukocyte extravasation was diminished in the absence of this cytokine.10,11 Similar to our findings, application of exogenous MK was able to restore the WT phenotype in these studies. Impaired leukocyte extravasation in the absence of MK had been described earlier in the literature,10,11 but it remained unknown at which step of the leukocyte recruitment cascade it was involved. To our knowledge, the present study provides the first evidence that MK is directly involved in PMN adhesion.
Only immobilized MK markedly mediated adhesion of both human and murine PMNs without further stimulation under static conditions suggesting that PMN adhesion was only fully induced in the presence of the immobilized form of the cytokine. In this model, PMN adhesion was dependent on CD18, as shown by using both a function-blocking anti-CD18 antibody and CD18−/− PMNs. Moreover, MK was also able to promote the induction of adhesion under physiological flow conditions in vitro. However, PMN-derived MK was dispensable for initial arrest. In contrast to the impact of MK on induction of adhesion, postadhesion strengthening was completely unaffected by MK. Thus, MK seems to specifically promote the initial induction of adhesion but may not be involved in postadhesion functions of PMNs. In accordance with the adhesion data, only immobilized MK, when combined with ICAM1, was able to markedly increase the number of β2 integrins in the high affinity state. Thus, MK may facilitate PMN adhesion by promoting the high affinity conformation of β2 integrins. The fact that slow rolling, which is mediated by the intermediate affinity conformation of β2 integrins6 was unaffected in the absence of MK suggests that MK specifically promoted the high affinity conformation of β2 integrins.
The mode of chemokine presentation in shaping cell adhesion and migration had previously been shown to be critical.45-47 Similar to our findings, only immobilized chemokines were essential for inducing the extended form of LFA-1 on lymphocytes, which, following ICAM1 binding, achieved full activation.48 In the case of MK, the cationic nature and heparin-binding domains of the molecule may allow its interaction with the negatively charged heparan sulfates of the glycocalyx, and thus, immobilized MK may be presented by the activated endothelium. As MK is not expressed on quiescent cells but upregulated during inflammation,18 only inflamed endothelium may promote the activation of β2 integrins on PMNs via MK. Thus, depending on the presence or absence of MK on endothelial cells in the microvasculature of an inflamed tissue, this mechanism may reinforce or attenuate PMN infiltration into the tissue by regulating intravascular adhesion, which may be of great importance for the balance between efficient host defense and protection from tissue damage by excessive PMN recruitment.
Various receptors including β1 integrins,21 LRP1,22 and heparan and chondroitin sulfate proteoglycans19,20 interact with MK. The exact mechanism through which these receptors exert their functions upon MK binding in different cell types and biological settings remains largely unknown. Using fluorescently labeled MK, its binding to PMNs was studied. Interestingly, TNF-α or CXCL1 enhanced MK binding, suggesting that the receptor for MK is upregulated in response to inflammatory stimuli. In contrast, MnCl2, which stabilizes the high affinity conformation of β2 integrins49 did not increase MK binding, suggesting that the high affinity state of β2 integrins does not favor MK binding. Abrogation of β1 or β2 integrin expression provided evidence that these receptors were not required for MK binding. Importantly, a blocking agent for LRP1 named LRPAP substantially inhibited MK binding, suggesting that LRP1 may represent a functional receptor for MK on PMNs. Furthermore, blockade of LRP1 attenuated the effect of immobilized MK to promote the high affinity conformation of β2 integrins indicating that LRP1 may act as a functional receptor for MK on PMNs. To date, no intracellular pathway linking LRP1 to β2 integrins has been identified. However, LRP1 directly interacts with the I-domain of Mac-1, and to a lesser extent with LFA-1.24 In the monocytic cell line U937, LRP1 was critically involved in β2 integrin clustering and subsequent adhesion.25 However, the molecular mechanism by which LRP1 modulates the affinity and avidity of β2 integrins and subsequent cell adhesion remains unknown. To our knowledge, a ligand for LRP1 mediating β2 integrin-dependent adhesion had not been proposed so far. Here, we report that MK may represent the critical factor that links LRP1 to β2 integrin function.
In summary, our findings demonstrate that PMN adhesion and subsequent extravasation were compromised during acute inflammation in the absence of MK. The immobilized form of the cytokine MK was able to mediate adhesion in vitro by promoting the high affinity conformation of β2 integrins. In this study, LRP1 may act as a functional receptor for MK on PMNs. Together, these findings may explain the decreased inflammatory response in the absence of MK providing evidence for a direct role of MK for PMN recruitment. Having identified MK as a critical player for PMN trafficking during acute inflammation, targeting this cytokine may provide a novel concept for an effective and cell-type-specific anti-inflammatory strategy.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tanja Vlaovic, Jennifer Truong, Susanne Bierschenk, Andrea Sendelhofert, Anja Heier, and Sabine Schaefer for their excellent technical assistance, as well as Dr Kyle Legate for fruitful scientific discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 914, project A2 [B.W.] and B1 [M.S.]) and the FöFoLe program of Ludwig-Maximilians-University.
Authorship
Contribution: L.T.W. and A.G. designed and carried out research, analyzed data, and wrote the manuscript; M.W., S.M.J., L.G., J.B., and F.P. carried out research; R.P., M.P., and J.M.-H. designed research; E.D. and M.S. designed research and analyzed data; and B.W. designed research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Walzog, Ludwig-Maximilians-University, Department of Cardiovascular Physiology and Pathophysiology, Walter-Brendel-Centre of Experimental Medicine, Schillerstrasse 44, 80336, Munich, Germany; e-mail walzog@lrz.uni-muenchen.de.

![Figure 3. Adhesion mediated by immobilized MK in vitro was CD18 dependent. (A-D) To measure adhesion of PMNs, cells were left untreated for control (w/o) or stimulated for 10 minutes at 37°C. Adhesion is expressed as a percentage of all cells added to poly-L-lysine (100%). (A) Adhesion of human or murine WT PMNs on immobilized FG left untreated (w/o) for control, or stimulated with soluble MK (10 ng/ml, 30 ng/ml, or 100 ng/ml), or TNF-α (100 ng/ml) (n = 18 [human]; n = 3 [murine]). (B) Adhesion of human or murine WT PMNs on immobilized FG or immobilized MK either left untreated (w/o) or stimulated with TNF-α (100 ng/ml) (n = 4 [human]; n = 4 [murine]). (C) Adhesion of murine WT PMNs on immobilized ICAM1 or MK. Cells were left untreated or preincubated with a CD18 function-blocking antibody (anti-CD18) for 20 minutes at RT. Subsequently, cells were left unstimulated (w/o) for control or stimulated with TNF-α (100 ng/ml) (n = 6 [ICAM1]; n = 9 [MK]). (D) Isolated CD18+/+ or CD18−/− murine PMNs on immobilized ICAM1 or MK. Diagrams show percentage of adhesion without stimulation (w/o) or after stimulation with TNF-α (100 ng/ml) (n = 3). (E) Induction of adhesion of murine WT PMNs under flow conditions (1 dyne/cm2). Microflow chambers were coated with P-selectin (10 µg/ml) and ICAM1 (12.5 µg/ml) alone (P-selectin + ICAM1), or combined with MK (P-selectin + ICAM1 + MK: 10 µg/ml). Data show the total number of adherent PMNs at indicated times (n = 5). (F) Adhesion of isolated MK+/+ and MK−/− PMNs under flow conditions. Microflow chambers were coated with P-selectin (10 µg/ml), ICAM1 (12.5 µg/mL), and CXCL1 (5 µg/ml) (n = 4). (G) Adhesion strengthening of adherent murine WT PMNs under gradually increasing shear stress (0.2-8.0 dyne/cm2). WT PMNs were seeded into microflow chambers coated with ICAM1 (ICAM1: 12.5 µg/ml) alone, or in combination with MK (ICAM1 + MK: 10 µg/ml), or CXCL1 (ICAM1 + CXCL1: 5 µg/ml) for 10 minutes before flow was applied as indicated. Adhesion strengthening was measured as the number of adherent PMNs in percent of initially adherent cells at 0.2 dyne/cm2 (100%) (n = 5). (A-E) Show mean ± SEM. *P < .05; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/12/10.1182_blood-2013-06-510875/4/m_1887f3.jpeg?Expires=1769212420&Signature=4nuTVbd-ZFmHUNmMZzyO-WtGjaBssxwHNnsGDSbgFxh69HTRkEUYlL0qbgjXpHL9CLE4a5GZnNHayTgKwldB11uN6gMbz9mFJQuY70Hrs2biAk9bPVgmTY-6-PTL0CGo1D5lW7RDg3ILoDSoWqkA8a4lKy5uO7frUMScL8olPQifyz4Vc-0YOw7XZE6w5TWMNZ5cDg5mONVpRIrGZj6kVQfKQTJQHlHNgfNR9bY3Vxol6~QrRIz3yEw~UP3LyOhu05pHNtOMavbEvAc68P63NiL5uQFjOSrz0n4Yz5WBMV1Bmyge8caLARsjjkii1IJwXRq-z3B6jq9Pri45TQTNjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)