In this issue of Blood, Bhagwat et al describe an elegant series of experiments showing that genetic deletion in the hematopoietic system of Janus kinase 2 (JAK2) abrogates initiation of myeloproliferative disease and substantial disease regression if deleted once disease is initiated.1
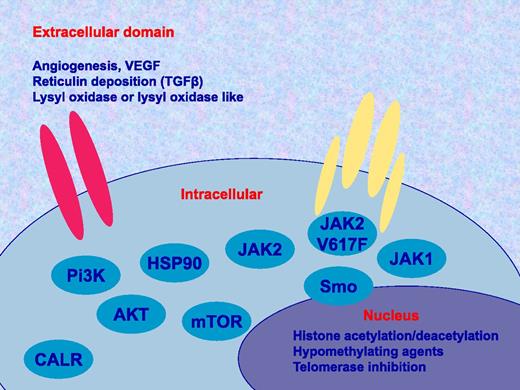
Potential therapeutic targets in MPN. There are many potential targets to study alone or in combination with JAK inhibitors such as ruxolitinib. These include in the extracellular domain angiogenesis or vascular endothelial growth factor (VEGF), transforming growth factor β (TGFβ), and lysyl oxidase or lysyl oxidase like; as well as cellular factors such as inhibitors specific for JAK2, V617F, JAK1, Hedgehog or smoothened (Smo) pathway, Pi3K/Akt/mTOR, HSP90, and calreticulin (CALR), in addition to drugs affecting histone acetylation, methylation of chromatin and the telomerase enzyme.
Potential therapeutic targets in MPN. There are many potential targets to study alone or in combination with JAK inhibitors such as ruxolitinib. These include in the extracellular domain angiogenesis or vascular endothelial growth factor (VEGF), transforming growth factor β (TGFβ), and lysyl oxidase or lysyl oxidase like; as well as cellular factors such as inhibitors specific for JAK2, V617F, JAK1, Hedgehog or smoothened (Smo) pathway, Pi3K/Akt/mTOR, HSP90, and calreticulin (CALR), in addition to drugs affecting histone acetylation, methylation of chromatin and the telomerase enzyme.
The description of the JAK2 V617F mutation in myeloproliferative neoplasms (MPNs) heralded many changes in the field including the potential promise of effective targeted therapy (a promise some would argue that current JAK inhibitors have failed to deliver), leading to questions concerning the validity of JAK2 as a target in these diseases. The work of Bhagwat and colleagues suggests that JAK2 is indeed a valid target and, in a series of additional experiments in mouse models (MPL515 and JAK2 V617F driven) and primary MPN cells, they also suggest that treatment with the heat shock protein 90 (HSP90) inhibitor PU H71, in addition with ruxolitinib, offers increased efficacy over ruxolitinib alone.
The therapeutic benefits of treatment with ruxolitinib, first in class JAK1 and JAK2 inhibitor, are both striking and surprising, with rapid reduction of massive splenomegaly, resolution of disease-related symptoms, and prolongation of survival; yet, this is achieved with minimal effect upon allele burden and marrow fibrosis.2,3 Significantly, there is a lack of understanding regarding which specific aspects of response are most important for particular clinical benefit. This does not reflect a weakness per se in the current trials but, more likely, a lack of an appropriate surrogate marker for longer-term end points. To this end, progress has been made in standardizing mutant JAK allele burden but this very process has highlighted many pitfalls. Candidate markers might be identified from a cytokine analysis of the COMFORT-2 trial4 or data with regard to T-cell function which in our hands has correlated with response.
Furthermore, while striking, the magnitude of benefit from ruxolitinib does not match that afforded by BCR/ABL inhibition in chronic myeloid leukemia. This reflects a number of issues: first, that none of the inhibitors yet developed are specific for mutant JAK2, and second, that JAK2 activation or its consequence may not be the only disease-initiating or -maintaining mechanism operating in these intriguing disorders. This was well illustrated by the recent reports of mutations in calreticulin in MPN almost specifically in those lacking the JAK2 exon 14 or MPL exon 10 mutations.5,6 The article from Nangalia demonstrated that patients with myelofibrosis, for example, have a mean of 15 somatic mutations.5
Concerning resistance to ruxolitinib, it is clear from the phase 3 studies (where 35% reduction in spleen volume, the primary end point, is concerned) that between 50% and 60% of patients continue to have benefit at ∼3 years but only 40% of patients achieved this end point initially. Suggesting firstly that the motivation for patients and clinicians to continue to use ruxolitinib is not wholly related to achieving 35% reduction in spleen volume. Second, there are a number of patients who do not respond initially (available data suggest these patients are rare) or who lose their response with time. The ability to recruit patients into second-line studies such as JAKARTA-27 suggests that this is the case. What about our understanding of the underlying mechanisms of resistance to ruxolitinib? Saturation mutagenesis screening carried out in mutant cells has demonstrated the presence of 5 “nonsynonymous” mutations in the JAK2 kinase domain which lead to not only resistance to ruxolitinib but also to cross resistance to other JAK2 kinase inhibitors.8 As yet, these mutations have not been seen in vivo. An additional mechanism of resistance is reactivation of the JAK/STAT pathway through heterodimerization of activated JAK2/JAK1 or TYK2 as discussed by Bhagwat et al and described in vivo by Koppikar et al.9
For complex conditions such as myelofibrosis, combination therapy may be more effective than use of a single agent either to overcome resistance or increase magnitude of benefit; it is also accepted practice in many hematological malignancies as first recognized by Farber when combining steroids with antifolate drugs in treating childhood acute leukemia. Ruxolitinib has a growing list of potential partners (see figure): those acting on epigenetic mechanisms such as histone deacetylase inhibitors or hypomethylating agents; it could also be combined with phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin inhibitors, Hedgehog inhibitors, second-generation immunomodulatory drugs, or Aurora A kinase inhibitors. A further combination strategy with stem cell transplantation10 is in active clinical trials though, interestingly, the “JAK ALLO” study has highlighted safety concerns of ruxolitinib used in this context which has yet to be fully understood. Bhagwat et al1 evaluate the concomitant use of the HSP90 inhibitor PU H71 and ruxolitinib based on their prior description that JAK2 is an HSP90 client protein and show this was highly effective in both mouse models and primary MPN cells thus providing a strong rationale for testing this combination in vivo.
Myelofibrosis is an extremely rare condition; a carefully considered approach will be necessary in selecting which combinations of drug therapies to test, and when, in the various disease stages. Furthermore, there is an urgent need for research to identify valid accurate surrogate end points in such studies where dissection of likely increased toxicity from meaningful clinical benefit is more challenging; otherwise there is a risk, as multiple trials emerge, that a beneficial effect may be missed.
Conflict-of-interest disclosure: C.N.H. has received research funding from Novartis Pharmaceuticals.