Key Points
Less than 60% of individuals who inherit a FAS mutation have a clinical manifestation of ALPS, implying a high carrier rate.
Major causes of morbidity and mortality in ALPS patients are sepsis following splenectomy and development of lymphoma.
Abstract
Autoimmune lymphoproliferative syndrome (ALPS) presents in childhood with nonmalignant lymphadenopathy and splenomegaly associated with a characteristic expansion of mature CD4 and CD8 negative or double negative T-cell receptor αβ+ T lymphocytes. Patients often present with chronic multilineage cytopenias due to autoimmune peripheral destruction and/or splenic sequestration of blood cells and have an increased risk of B-cell lymphoma. Deleterious heterozygous mutations in the FAS gene are the most common cause of this condition, which is termed ALPS-FAS. We report the natural history and pathophysiology of 150 ALPS-FAS patients and 63 healthy mutation-positive relatives evaluated in our institution over the last 2 decades. Our principal findings are that FAS mutations have a clinical penetrance of <60%, elevated serum vitamin B12 is a reliable and accurate biomarker of ALPS-FAS, and the major causes of morbidity and mortality in these patients are the overwhelming postsplenectomy sepsis and development of lymphoma. With longer follow-up, we observed a significantly greater relative risk of lymphoma than previously reported. Avoiding splenectomy while controlling hypersplenism by using corticosteroid-sparing treatments improves the outcome in ALPS-FAS patients. This trial was registered at www.clinicaltrials.gov as #NCT00001350.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) usually presents in childhood with nonmalignant splenomegaly and lymphadenopathy along with expanded CD4 and CD8 negative or double negative T-cell receptor αβ+ T cells (DNTs), chronic multilineage cytopenias, and an increased risk for developing B-cell lymphomas due to failed lymphocyte apoptosis.1-8 Deleterious heterozygous mutations in the FAS gene are the most common cause of this condition, which is termed ALPS-FAS. ALPS-FAS is caused by genetic abnormalities in FAS (TNFRSF6/APO1/CD95), a member of the TNF receptor superfamily that induces apoptosis9-12 (Figure 1). The primary required criteria for ALPS-FAS include chronic lymphadenopathy or splenomegaly and elevated DNTs with a normal or elevated lymphocyte count and a FAS gene mutation that impairs apoptosis1,2,7 (Table 1). DNTs, which accumulate with either somatic or germ-line FAS mutations, are important mediators of disease.8,9 Somatic FAS defects resulting in ALPS (ALPS-sFAS) were reported previously.13,14 A recent publication presented 90 ALPS-FAS patients but did not address the diagnostic or pathogenic roles of apoptosis defects or biomarkers.15 Although our center has comprehensively studied this disease for the last 20 years, a full accounting of the pathophysiology, natural history, and management has not been reported.
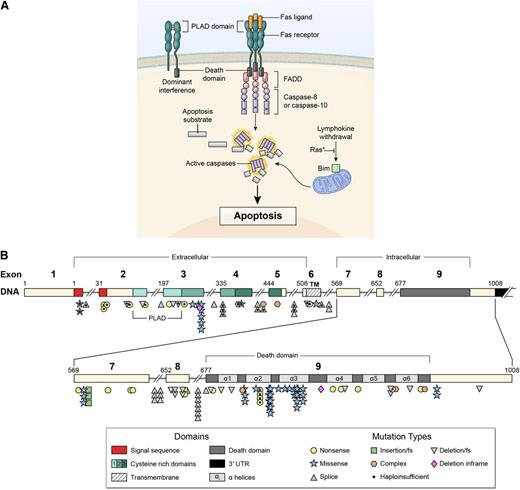
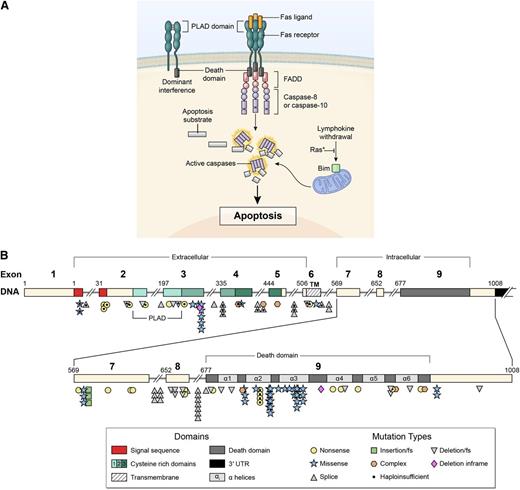
Signal transduction by the FAS receptor whose gene is mutated in ALPS as detailed in the Introduction. (A) Schematic of the signaling complex formed after the engagement of FAS by FAS ligand that leads to apoptosis. Shown at the top left is an example of a mutant FAS receptor chain lacking the death domain bound to a wild-type chain through the PLAD, which prevents it from signaling causing dominant interference. (B) Diagram showing the intron-exon structure of the FAS gene with delineation of exons coding for the extracellular, transmembrane, and intracellular portions of the protein incorporating the death domain and the location and types of mutations associated with ALPS-FAS. It is notable that R250 in the α2 helical region of the death domain is the most frequently altered residue and exhibits haploinsufficiency due to reduced Fas surface expression, as well as dominant interference.22,23
Signal transduction by the FAS receptor whose gene is mutated in ALPS as detailed in the Introduction. (A) Schematic of the signaling complex formed after the engagement of FAS by FAS ligand that leads to apoptosis. Shown at the top left is an example of a mutant FAS receptor chain lacking the death domain bound to a wild-type chain through the PLAD, which prevents it from signaling causing dominant interference. (B) Diagram showing the intron-exon structure of the FAS gene with delineation of exons coding for the extracellular, transmembrane, and intracellular portions of the protein incorporating the death domain and the location and types of mutations associated with ALPS-FAS. It is notable that R250 in the α2 helical region of the death domain is the most frequently altered residue and exhibits haploinsufficiency due to reduced Fas surface expression, as well as dominant interference.22,23
The FAS receptor is homotrimeric and activated by the cognate FAS ligand (FASL), another homotrimeric protein complex homologous to TNF10,11,16 (Figure 1A). Following stimulation, the intracellular death domain (DD) portion of FAS nucleates an extended helical complex of the FADD adaptor protein and caspases-8 and −10.16-19 These caspases undergo proteolytic autoprocessing and cleave, in a signaling cascade, downstream effector caspases and other targets, leading to apoptosis.17 The FAS gene contains nine exons spanning 26 kb on chromosome 10q24.120 (Figure 1B). The first 5 exons encode the extracellular portion containing 3 cysteine-rich domains that control receptor trimerization and FASL binding. Exon 6 represents the transmembrane domain (TM), and the intracellular portion is encoded by exons 7 through 9. The FAS DD encoded by exon 9 is 85 amino acids long and critical for apoptosis signaling.10,16,19,20 ALPS-FAS is most frequently caused by heterozygous mutations that generate mutant FAS proteins, often with defective DDs.1,10 The defective FAS chains associate with wild-type chains via the pre-ligand assembly domain (PLAD), resulting in functionally defective receptor trimers, a phenomenon termed “dominant interference”1,10,21 (Figure 1A). Less frequently, heterozygous mutations cause decreased FAS protein and haploinsufficiency.22-24 Mutations in genes encoding FASL, FADD, and CASP10 also cause ALPS termed ALPS-FASL, ALPS-FADD, and ALPS-CASP10, respectively25-30 (Figure 1A). Germ-line mutations in CASP8 or somatic mutations in NRAS and KRAS cause ALPS-related syndromes with distinct clinical phenotypes.31-33 Importantly, FAS mutations are associated with in vitro lymphocyte apoptosis defects but may show variable clinical penetrance, and this mechanism has not been fully defined.1,2,8-11 Here, we describe the comprehensive clinical findings and molecular and laboratory assessments of 150 ALPS-FAS patients and 63 healthy mutation-positive relatives (HMPRs) to broaden the current understanding of the diagnosis and management of ALPS-FAS.
Methods
Cohort description
The National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), ALPS program has studied patients and family members through mail-in samples and direct physical evaluation at the NIH Clinical Center since 1993 and has identified 120 families with 95 unique FAS mutations (http://www.ncbi.nlm.nih.gov/lovd/home.php?select_db=FAS) (Figure 1B). Of 744 individuals screened, 415 (56%) harbored FAS mutations (M+) and 329 (44%) were negative (M−). Remarkably, among the 415 M+ individuals, 240 (58%) had ALPS symptoms and 175 (42%) were HMPRs with no disease manifestations. We found that the disease was penetrant in 149 of 215 (69%) M+ males and 91 of 200 (46%) M+ females. We report here the natural history of a subset of 150 ALPS-FAS patients (78 probands and 72 affected relatives) and 63 HMPRs evaluated at the NIH Clinical Center since 1993 (Table 2). Eighty-five percent of these families had all of their first-degree relatives screened.
Clinical data were collected from 1993 to July 2011. Vital status data were collected through March 1, 2013. All participants underwent informed consent process and enrolled on an institutional review board–approved National Institute of Allergy and Infectious Diseases, NIH research protocol. This study was conducted in accordance with the Declaration of Helsinki.
Clinical assessment
Medical records were reviewed for symptom onset, cytopenias, lymphadenopathy and spleen assessments, autoimmune manifestations, lymphoma and other cancers, treatment regimens, date of diagnosis, and deaths. Pedigrees were prepared for each family. Lymphadenopathy and splenomegaly were assessed longitudinally by physical examination and computerized tomography (CT) and scored as grade 1 to 4 using consistent in-house practices and published criteria.34-36 Spleen size and volume were calculated from manually segmented axial slices from CT images. Patients developing a grade 3 or 4 cytopenia within 2 years of the onset of their symptoms were considered to have severe disease.
Laboratory assessments
Cytopenias were graded from complete blood counts by using National Cancer Institute Common Toxicity Criteria (version 3.0). Serum vitamin B12 and immunoglobulin levels were determined by standard methods. For serum vitamin B12, serial dilutions were performed to quantify high levels (>4000 pg/mL). Interleukin (IL)-10 and soluble FAS ligand (sFASL) were determined using Quantikine (R&D Systems, Minneapolis, MN) or enzyme-linked immunosorbent assay kits (Enzo Life Sciences, Farmingdale,NY). Flow cytometry data and apoptosis assays were quantified using published methods.1,10,21,37
Molecular biology assessment
Pathological assessment
Available lymph node, spleen, liver, and bone marrow biopsies were reviewed by the hematopathologists at the National Cancer Institute. Immunophenotypic studies were performed on paraffin sections using the Avidin Biotin complex with diaminobenzidine as chromogen for the immunoperoxidase immunoperoxidase technique with antibodies against CD3, CD45RO, CD4, CD8, and CD20 as previously described. An expanded lymphocyte panel was used if lymphoma was suspected. Most lymphoma diagnoses were made locally; however, biopsies were reviewed at the National Cancer Institute. Clonality was evaluated by flow cytometry and/or evaluation of lymph node tissue for T-cell receptor and IgH gene rearrangements by polymerase chain reaction.39,40
Statistical methods
Follow-up time (years) was defined from symptom detection to either the date of final data extraction or death, whichever occurred first, and represents both retrospective and prospective evaluations. The date of symptom detection was defined as the first record of lymphadenopathy, splenomegaly, or a grade 3 or 4 cytopenia (neutropenia, anemia, or thrombocytopenia). Kaplan-Meier analyses examined the age at splenectomy, lymphoma, sepsis-free survival, relapse of cytopenia postsplenectomy, and overall survival. Cox regression was used to identify patient factors associated with increased risk of events, using robust standard errors to account for correlation within family clusters. To quantify the incidence of specific neoplasms, we compared the observed numbers of cancers among 150 ALPS-FAS patients to the expected number (O/E ratio) after accounting for age, gender, birth cohort, and race, based on the experience of the US Surveillance, Epidemiology, and End Results Program (SEER), November 2012 Submission (1973-2010), released April 2013 (www.seer.cancer.gov). Statistical analyses used SAS (version 9.3), R (version 2.14.0), and SEER*Stat software (version 8.0.4.) (SEER.cancer.gov/seerstat). All tests were 2-sided, and significance was tested at 0.05.
Results
Patient demographics
Key patient demographics that form the basis of this report are presented in Table 3. All patients harbored heterozygous germ-line pathogenic FAS mutations, with the exception of 1 with a homozygous mutation. The HMPRs had an in vitro apoptosis defect but no lymphadenopathy, splenomegaly, or cytopenias. Our subjects belonged to 78 families from 30 states and the District of Columbia in the United States and 4 other countries (Switzerland, Norway, Canada, and Australia). Demographically, they were 92% white non-Hispanic, 3% Hispanic, and 5% black. There were 97 males (65%) and 53 females (35%) consistent with a previous report.15 The median age at onset of disease was 2.7 years, but symptoms could be appreciated at birth in many cases. The median follow-up time was 13.5 years with a range of 1.3 to 55.7 years. Only 1 patient had <2 years of follow-up. Fifty-six patients (37%) had a severe disease phenotype. The probands had more severe disease compared with affected relatives (54% vs 19%), although this likely reflects ascertainment bias. The male/female ratio was 1.6 in ALPS-FAS patients and 0.58 among HMPRs (Table 3).
Molecular genetics
We found that most FAS mutations affected the intracellular portion (n = 110, 73%), usually the DD (n = 70), whereas 21% (n = 31) had extracellular defects, and 6% (n = 9) had TM defects. Most intracellular mutations outside the DD led to loss of DD by premature stop or frame shift (Figure 1B). Among the probands who had parental testing, 7 had a de novo germ-line mutation, 36 had maternal inheritance, 27 had paternal inheritance, and 1 was homozygous. Most FAS gene defects were missense mutations that produced a defective protein that exerted a dominant-negative effect on signaling1,10,21 (Figure 1). However, we also observed mutations causing FAS haploinsufficiency by abrogating FAS protein expression.22-24 We noted that dominant-interfering mutations preserve the PLAD, which mediates the association of defective and normal FAS chains19 (Figure 1). By contrast, 23 mutations that remove the PLAD generally caused haploinsufficiency.23
Lymphoproliferation
Lymphadenopathy and splenomegaly were seen in 97% and 95% of our patients, respectively. There were no cases of splenic rupture despite massive spleen size in some children. Spleen volumes measured by CT scans for 35 patients (both adults and children) ranged from 389 to 1858 mL (median, 968 mL) compared with reported normal adult spleen volumes of 237 (+78) mL.34 In 1 patient, splenic enlargement was clinically first noticed at age 52 due to thrombocytopenia and anemia, eventually leading to diagnostic splenectomy. Among 115 patients with ≥1 CT scan evaluation of lymph nodes, 13% had grade 4 (visible adenopathy), 48% had grade 3 (>2 cm), 27% had grade 2 (1-2 cm), and 12% had grade 0-1(<1 cm) lymphadenopathy. There were 37 subjects with ≥2 lymph node evaluations 5 years apart by CT scan, and we did not observe significant trends of increasing or decreasing lymphadenopathy, but our general impression was that lymphadenopathy improved with age in adulthood.
Laboratory parameters and biomarkers
All ALPS-FAS patients had elevated DNT cells with a normal or elevated lymphocyte count, which was a required diagnostic criterion (Table 4). The median percentage of DNTs was 5.9%, which is substantially higher than other related conditions in which DNTs may be noted. Serum vitamin B12 proved to be a reliable and readily available biomarker that correlates with lymphoproliferation, although the molecular mechanism of its elevation is unknown.41,42 Patients exhibited serum B12 as high as 47 920 pg/mL (median value, 2798 pg/mL compared with a normal value of <1320 pg/mL). We also observed consistently elevated sFASL and IL-10 (Table 4). These biomarkers may be used to make a presumptive diagnosis of ALPS-FAS prior to molecular testing.41,43-45 We found elevated IgG, IgA, and IgM in 58%, 45%, and 10% of 143 patients tested, respectively (data not shown). An elevated absolute monocyte count of >1.0 K/μL was seen in 38% of the patients, whereas 24% had an absolute eosinophil count >0.75 K/μL on ≥1 occasion. We also tested for direct antiglobulin test (DAT), rheumatoid factor (RF), anticardiolipin antibody, antinuclear antibody, extractable nuclear antigen antibodies, and antithyroid autoantibodies. Although DAT and RF were positive in 40% and 32% of patients, respectively; patients with a positive DAT often did not exhibit hemolytic anemia and none with a positive RF had symptoms of rheumatoid arthritis. antinuclear antibody and anticardiolipin antibody were frequently positive at low levels, and antithyroid antibodies were detected in 7% of the patients.
Apoptosis defects
We carried out FAS-mediated apoptosis assays on all probands at the initial NIH Clinical Center visit and in a subset of HMPRs and mutation-negative relatives. We found that the median and mean percentage of cells undergoing apoptosis were significantly lower (P < .0001) in both ALPS patients (5 and 13%) and HMPRs (21% and 24%) compared with healthy mutation-negative relatives (68% and 64%). Notably, the apoptosis defect was modest in HMPRs, although it was significantly less severe in HMPRs compared with patients (P < .03; supplemental Figure 1 on the Blood Web site).
Histopathology
Histopathology revealed characteristic extrafollicular expansion of DNTs in lymphoid tissue in many cases, distinguishing ALPS-FAS from other lymphoproliferative disorders.40 Lymph node and spleen tissue were available for evaluation in 64 and 25 patients, respectively. Follicular and paracortical hyperplasia were notable in 76% (n = 49) and 67% (n = 43) of the lymph node samples, respectively. Paracortical DNT expansion was confirmed in 41% (n = 26) of specimens. In 2 patients (3%), proliferation was extensive and effaced the nodal architecture. We observed S100+ histiocytes characteristic of Rosai Dorfman disease in 27% (n = 17) and progressive transformation of germinal centers in 12% (n = 8) without clonal T- or B-cell proliferation.39,46 Occasionally, expansion of the splenic red pulp due to atypical T-cell proliferation, extramedullary erythropoiesis in patients with anemia, and moderate degrees of follicular hyperplasia, with increased DNTs and polyclonal plasmacytosis, was noted.
Lymphoma
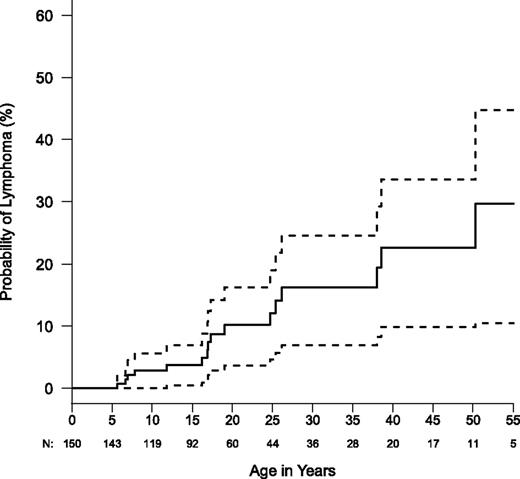
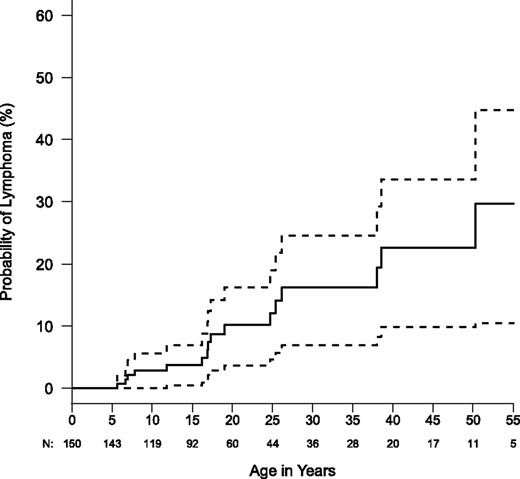
We found that ALPS-FAS patients had a greater relative risk of lymphoma than previously described.6 Eighteen cases of lymphoma and 1 nonlymphoid hematopoietic malignancy were noted in this entire cohort of 150 ALPS FAS patients and 63 HMPRs (Table 5). The age at diagnosis ranged from 5 to 60 years (median, 18 years). The male to female ratio was 14:4. We observed 16 B-lineage lymphomas in ALPS-FAS patients. This included 10 cases of Hodgkin’s lymphoma in 150 ALPS-FAS patients compared with 0.067 expected in the general population, giving an O/E of 149 (95% confidence interval [CI] = 71-274). Six cases of non-Hodgkin lymphoma were observed in ALPS-FAS patients compared with the 0.099 expected (O/E = 61; 95% CI = 22-132). Both O/E ratios were highly significant. Standardized incidence ratio values for ALPS-FAS patients are 149 and 61 for Hodgkin and non-Hodgkin lymphoma, respectively, in the current report vs 51 and 14, respectively, in the 2001 report.6 This difference may be related to the cumulative increase of lymphoma with age and accrual of additional events and person-years (Figure 2). Systemic symptoms associated with lymphoma presentation included fever, fatigue, weight loss, loss of appetite, and/or sudden focal lymph node enlargement. Only 25% of the ALPS-FAS patients with lymphoma had a severe disease phenotype. All lymphoma patients had dominant-interfering FAS mutations affecting the DD except for one affecting the extracellular domain. Because haploinsufficient patients may constitute almost a fifth of our population, the incidence of lymphoma is even higher among patients with dominant-interfering mutations, implying that more disruptive FAS mutations may confer a greater propensity to lymphomagenesis. We also observed that 2 HMPRs (both siblings from the same family) developed lymphoma. Nonlymphoid malignancies also occurred in our cohort, but these were not significantly increased compared with reported population norms. One patient with Hodgkin’s lymphoma developed histiocytic sarcoma as a second malignancy.46 Nonhematopoietic malignancies included a squamous cell carcinoma of the tongue and a pleomorphic adenoma.
Kaplan-Meier curve for lymphoma risk in ALPS-FAS (N = 150). Dashed lines show the 95% confidence bands. Total numbers are not censored and numbers of patients still at risk are shown below x-axis.
Kaplan-Meier curve for lymphoma risk in ALPS-FAS (N = 150). Dashed lines show the 95% confidence bands. Total numbers are not censored and numbers of patients still at risk are shown below x-axis.
Multilineage cytopenias and their management
Recurrent multilineage cytopenias were common in ALPS patients, with 104 (69%) individuals having ≥1 grade 3 or 4 cytopenia. The median age of first cytopenia was 5.6 years (range = <1-53 years). Grade 4 anemia occurred in 36% of patients (n = 54), and grade 4 thrombocytopenia and neutropenia were noted in 39% (n = 59) and 37% (n = 56) of the patients, respectively. We found that 21% (n = 31) had a single lineage cytopenia, 23% (n = 35) had bilineage cytopenias, and 25% (n = 38) had trilineage cytopenias. There were no gender differences. Although many patients presented with cytopenias as their first symptom, in others, the median time from lymphadenopathy or splenomegaly to the first episode of cytopenia was 1.9 years (range, 0-37 years). Fifty-nine percent of the patients (n = 88) were treated for cytopenias, but their frequency appeared to decline with age. Of those, 90% (n = 79) received pulse corticosteroids, often the most effective immediate intervention.47 However, only 6 patients responded long term to corticosteroids alone. Most patients required multiple agents, with the maximum and median number of different therapeutic agents being 9 and 3, respectively. We achieved success with long-term (>1 year) immunosuppression using mycophenolate mofetil (n = 23) and sirolimus (n = 2) in this cohort. Other therapeutic interventions include intravenous immunoglobulin, rituximab, vincristine, cytoxan, hydroxycholoroquine, granulocyte colony-stimulating factor, and WinRho, although 1 individual developed severe hemolysis after WinRho.
Splenectomy and its consequences
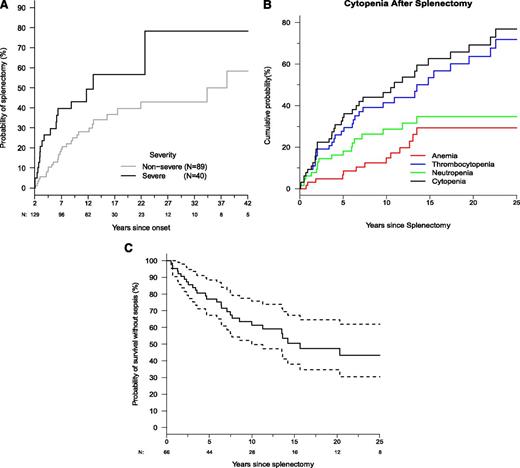
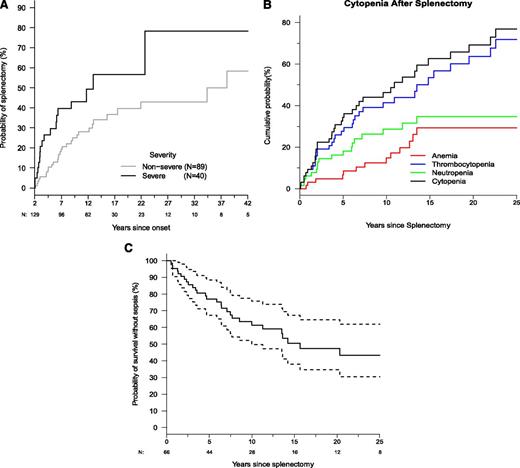
Many patients (40%; n = 66) enrolled prior to the development of steroid-sparing immunosuppressive strategies underwent splenectomy to manage cytopenias. There was a 25% probability of splenectomy 4.5 years after symptom onset and an ∼50% probability within 18 years, with age at splenectomy ranging from 6 months to 52 years (Figure 3A). Severe disease was associated with increased likelihood of splenectomy (95% CI: 1.60-4.14). Prior to splenectomy, 74% of patients had severe cytopenias: anemia (55%), thrombocytopenia (45%), and neutropenia (44%), which often presented simultaneously. Postsplenectomy, thrombocytopenia predominated with an estimated probability of 60% (Figure 3B). The likelihood of cytopenia relapse after splenectomy was nearly 30% by 4 years and exceeded 70% by 20 years. Because the median time between onset of symptoms and splenectomy was only 4 years compared with a median postsplenectomy follow-up of 12 years, we compared pre- and postsplenectomy times using McNemar’s test. This showed a significant reduction in anemia (P < .0001) after splenectomy, but neutropenia or thrombocytopenia recurred and persisted.
Splenectomy is a common intervention that leads to sepsis but does not prevent cytopenias. (A) Kaplan-Meier curve for time to splenectomy by severity classification (N = 150). Dashed lines show the 95% confidence bands. (B) Kaplan-Meier curve for time until first occurrence of cytopenia after splenectomy. Time to first cytopenia of any kind is shown, and time until first occurrence of anemia, neutropenia, and thrombocytopenia are shown. (C) Kaplan-Meier curve for sepsis-free survival after splenectomy (N = 66). Dashed lines show the 95% confidence bands. Total numbers are not censored and still at risk are shown below x-axis.
Splenectomy is a common intervention that leads to sepsis but does not prevent cytopenias. (A) Kaplan-Meier curve for time to splenectomy by severity classification (N = 150). Dashed lines show the 95% confidence bands. (B) Kaplan-Meier curve for time until first occurrence of cytopenia after splenectomy. Time to first cytopenia of any kind is shown, and time until first occurrence of anemia, neutropenia, and thrombocytopenia are shown. (C) Kaplan-Meier curve for sepsis-free survival after splenectomy (N = 66). Dashed lines show the 95% confidence bands. Total numbers are not censored and still at risk are shown below x-axis.
Among the 66 splenectomized patients, 41% (n = 27) had ≥1 episode of sepsis, and among the 27 patients who developed sepsis, 6 died due to sepsis. We found that within 6.4 years and even until 20 years after spleen removal, there was a 25% and 50% risk, respectively, of sepsis, indicating this is an important long-term complication (Figures 3 and 4). We also found that splenectomy at a younger age was associated with an increased risk of sepsis. Every 5-year increase in age at splenectomy was associated with a 33% decrease in the risk of sepsis or death (95% CI: 0-55%; P = .05). The most common bacteria causing septicemia were Streptococcal pneumoniae seen in 70% (n = 19) of patients, 2 patients had Haemophilus influenza bacteremia, 6 patients developed meningitis involving either Streptococcal pneumoniae or Nisserial menigitidis; Capnocytophaga cynodegmi bacteremia occurred in 1 patient, and 1 patient died of Edwardsiella tarda. Ten patients had >1 episode of sepsis, with 1 patient having 8 episodes of sepsis. At the time of sepsis development, 60% were reportedly on a prescribed daily antibiotic for prophylaxis for asplenia; however, compliance was uncertain. Eight of 13 individuals developed pneumococcal sepsis despite immunization with Prevnar. Clearly overwhelming postsplenectomy sepsis was a major cause of morbidity and mortality.
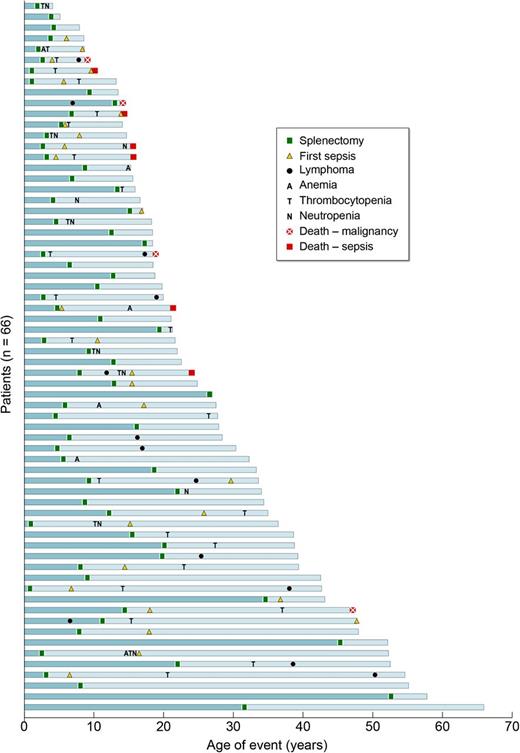
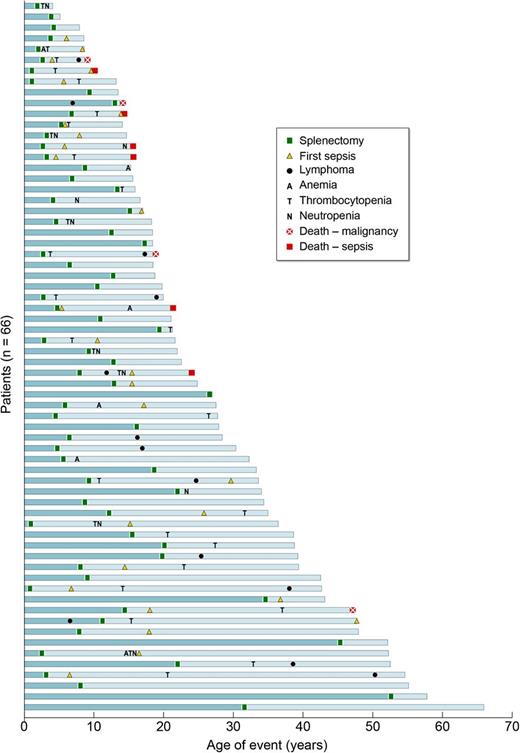
Causes and consequences of splenectomy in ALPS-FAS. Outcomes for all 66 patients undergoing splenectomy are shown. Note that each blue bar represents the timeline of events in 1 patient’s lifetime, and all the bars are stacked from the oldest at the bottom to the youngest patient in the cohort at the top. All 6 deaths due to sepsis were seen in patients who underwent splenectomy at a younger age (<10 years).
Causes and consequences of splenectomy in ALPS-FAS. Outcomes for all 66 patients undergoing splenectomy are shown. Note that each blue bar represents the timeline of events in 1 patient’s lifetime, and all the bars are stacked from the oldest at the bottom to the youngest patient in the cohort at the top. All 6 deaths due to sepsis were seen in patients who underwent splenectomy at a younger age (<10 years).
Healthy mutation-positive relatives
We evaluated 63 HMPRs from 44 families who shared mutations with affected family members but had no lymphadenopathy, splenomegaly, or autoimmunity and did not meet the criteria for an ALPS diagnosis (Table 2). These included 40 females and 23 males with a median age of 46 years (range, 5-92 years) at the time of their evaluation. FAS mutations affecting the intracellular, extracellular, and transmembrane portion of the protein were noted in 29, 29, and 5 individuals, respectively. As noted above, apoptosis defects in HMPRs were nearly as severe as in patients. Some HMPRs also had modest elevations of biomarkers: serum IgG (1/62), serum vitamin B12 (7/57), elevated α/β DNT (13/62), sFASL (34/63), and IL-10 (9/63). In those with elevated biomarkers, the serum vitamin B12 median was 682 pg/mL (range, 184-5348), α/β DNT median was 2.2% (range, 1.7-5.9%), the median for sFASL was 306 pg/mL (range, 208-1414 pg/mL), and for IL-10, the median was 40 pg/mL (range, 21-70 pg/mL).
Survival
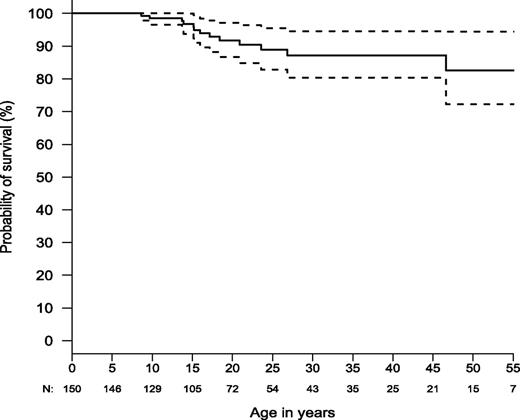
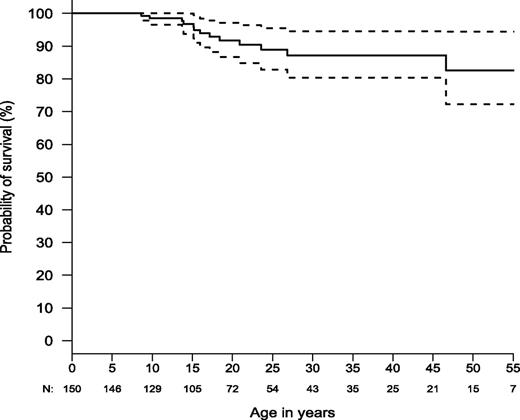
The prognosis for ALPS-FAS is good for survival to adulthood. Estimated survival was ∼85% by age 50 (N = 150; Figure 5) compared with healthy non-ALPS individuals in the general population, with an expected survival in the range of 93% to 95% at age 50 (http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_03.pdf). Nine percent (n = 13) of the 150 patients died during the study. Six patients died of documented or probable bacterial sepsis after splenectomy. One died of fungal infection (Aspergillosis) following immunosuppressive medications for refractory autoimmune hemolytic anemia. Three died as a result of lymphoma. One died during hematopoietic stem cell transplant for histiocytic sarcoma and another from metastatic squamous cell cancer of the tongue. One patient died of a gunshot wound.
Kaplan-Meier curve for overall survival (N = 150). Dashed lines show the 95% confidence bands. Total number alive and not censored are shown below x-axis.
Kaplan-Meier curve for overall survival (N = 150). Dashed lines show the 95% confidence bands. Total number alive and not censored are shown below x-axis.
Discussion
Herein we report our 20-year experience with a large cohort of ALPS-FAS patients, which extends earlier reports on this disease showing that the most severe complications are childhood-onset multilineage cytopenias and lymphoma.4,15,47-49 Our molecular genetic analyses indicate that the predominant pathogenetic mechanism, especially for the predisposition to lymphoma, is dominant interference with the apoptosis signaling complex by abnormal FAS proteins.1,10,50 This mechanism fits well with the fact that our population is largely outbred, and the mutations were heterozygous in all cases except one. However, we also report new and important observations: a large number of HMPRs, the reliability of blood biomarkers such as vitamin B12 and sFASL levels to substitute for molecular analyses, the clinical futility and unwarranted complications of splenectomy, and a significantly higher incidence of lymphoma than previously documented.
We now establish that the clinical penetrance is <60% among individuals carrying a pathogenic FAS mutation, with lower penetrance for those harboring extracellular mutations.24 HMPRs exhibited apoptosis defects almost as severe as patients but biomarkers such as DNTs, sFASL, and IL-10 showed slight if any elevations. Thus, FAS mutations causing cellular apoptosis abnormalities are not sufficient to cause clinical ALPS. We also documented that the male/female ratio was significantly higher in patients compared with HMPRs. Thus, male predominance among clinically affected individuals contrasts with the preponderance of women among individuals developing other autoimmune conditions. However, we did not notice statistically significant difference in disease severity based on the gender. Similarly, while examining the pedigrees, we saw no difference in penetrance in terms of maternal or paternal transmission of the FAS gene mutation. It has been recently reported that somatic loss of heterozygosity in addition to germ-line haploinsufficiency may lead to full-blown ALPS in 1 of 12 family members with FAS start codon mutation.51 Therefore, environmental factors or other genetic abnormalities, especially those occurring selectively in DNTs, have a significant impact on clinical penetrance and should be sought in future analyses.
We originally introduced biomarkers as an inexpensive means to presumptively diagnose ALPS-FAS, which is especially useful when molecular analysis is unavailable. Subsequent investigation has shown that serum vitamin B12 and sFASL can reliably identify ALPS-FAS patients; however, these biomarkers may also be elevated in other conditions including common variable immunodeficiency.4,41,43,45 The precise factors underlying disease pathogenesis remain undefined but appear to relate to the expansion of DNTs, which are significantly elevated in all patients but much lower in HMPRS. Notably, somatic FAS mutations within DNTs are a phenocopy of germ-line ALPS-FAS.4,14,15 How mutant DNTs contribute to multilineage cytopenias is not clear, but may involve the production of IL-10 and sFASL. Recently, we reported a simplified flow cytometric apoptosis assay that can be carried out on T lymphocytes and DNTs immediately ex vivo without the need for in vitro cultivation, which could simplify and decrease the cost of diagnosis.52
Chronic, refractory multilineage cytopenias due to splenic sequestration and autoimmune destruction were the most frequent cause of morbidity. This led to surgical splenectomy in 66 individuals (Figure 4). However, splenectomy does not prevent the recurrence of cytopenias (Figure 3B) and, if anything, increases DNTs (data not shown) and carries significant risk of sepsis as observed in other studies of splenectomy complications.53 The fact that 41% (27/66) of the splenectomized patients in our cohort had ≥1 episode of postsplenectomy sepsis, and 6 of them have died due to overwhelming postsplenectomy sepsis profoundly reinforces the recommendation of avoiding splenectomy and managing chronic cytopenias pharmaceutically.47,54,55
The other major adverse event has been the development of lymphoma as we found the risk is ≥3 times higher than previously reported.4 The improved estimate not only reflects our patients’ older age and longer follow-up but also a more refined calculation of expected values accounting for age, gender, race, and calendar year. Despite the fact the DNTs were the major cell type to accumulate in the circulation and lymph nodes, the lymphomas were invariably of B-cell origin. Interestingly, 2 HMPRs from the same family also developed lymphoma as older adults, but it is unclear whether this is related to mutant FAS. Lymphoma has not been observed in patients with FAS haploinsufficiency, indicating that the risk for patients with dominant interfering mutations is even higher than the overall risk calculated on the basis of all ALPS-FAS patients. Thus, FAS acts as a potent molecular restraint to lymphomagenesis most likely by suppressing Epstein-Barr virus–infected B cells. ALPS-FAS patients should be monitored for systemic symptoms and clinical evidence of sudden focal enlargement of lymph nodes before subjecting them to further workup, including radiological imaging and excisional biopsy to rule out lymphoma.
In summary, the prognosis for ALPS-FAS is good and depends on steroid-sparing management of cytopenias with mycophenolate mofetil or sirolimus, splenectomy avoidance, and vigilance for lymphoma. It is encouraging that the time from symptom onset to diagnosis among probands has declined since ALPS-FAS was first described in 1995, indicating faster diagnosis following improved index of suspicion. Indeed, time to diagnosis was 5 years shorter for those diagnosed after February 2002 (median date for the cohort) compared with before (P = .02). We hope the new insights in this report will facilitate rapid diagnosis and successful treatment of ALPS-FAS in the community.56,57
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all patients and their families for traveling to NIH and participating in this natural history study. The authors thank referring physicians and patients’ local caregivers for sharing their clinical records. The authors thank Fred Gill, Joao Bosco Oliveira, Kennichi Dowdell, Janet Dale, Faith Dugan, Francine Thomas, Hyesun Kuehn, Alan Hoofring, Alan Remaley, Michael Curry, Neelam Giri, Karen Thatcher, Yanmei Wang, Jean Tretler, and Lisa Barnhart, as well as the ALPS clinical team including Patricia Aldridge, Debbie Rawson, and Elaine Smoot, for providing clinical and laboratory support. The authors also thank Jeffrey Cohen and Stephen Holland for their support and encouragement. This study was initiated by Stephen E. Straus (1946-2007) and is dedicated to his memory.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and the NIH Clinical Center. This project has been funded in whole or in part with federal funds from National Cancer Institute grant HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: S.P., V.K.R., J.M.P., and M.J.L. designed the research; S.P., V.K.R., J.M.P., M.J.L., A.P.H., J.E.N., K.P., R.L.H., J.D., L.F., S.P., E.S.J., and B.L. collected data; S.P, V.K.R., J.M.P., A.P.H., E.S.J., T.A.F., and M.J.L. analyzed and interpreted data; P.A.S., A.S., G.J., and P.S.R. performed statistical analysis; S.P, V.K.R., P.A.S., and M.J.L. wrote the manuscript; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: V. Koneti Rao, Building 10, Room 12C106, National Institute of Allergy and Infectious Diseases, NIH, 10 Center Dr, MSC-1899, Bethesda, MD 20892-1899; e-mail: koneti@nih.gov.
References
Author notes
V.K.R. and M.J.L. contributed equally to this work.