In this issue of Blood, de Smith et al, in a new case-control association study of polymorphisms in key immunologic genes KIR and HLA, provide further evidence that genetic variation may contribute to differences in pediatric acute lymphoblastic leukemia (ALL) incidence between Hispanic and non-Hispanic ethnic groups.1
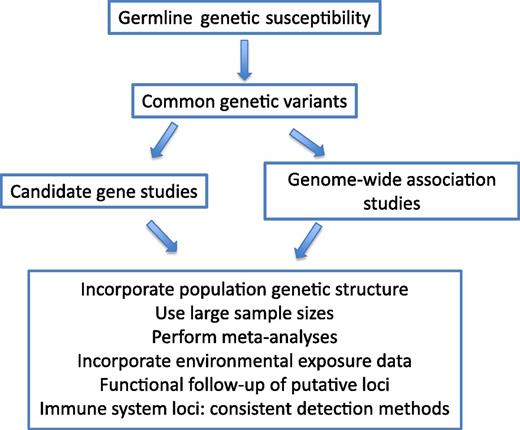
Approaches to understanding the genetics underlying ethnic disparities in ALL etiology and outcome.
Approaches to understanding the genetics underlying ethnic disparities in ALL etiology and outcome.
It has long been recognized that there is a higher incidence of ALL in children of Hispanic ancestry (24.9 per million person-years) than in non-Hispanic whites (16.6 per million).2 Children of Hispanic ancestry also have significantly worse 5-year survival from ALL compared with non-Hispanic whites (77% vs 87%).3 The reasons for these differences between ethnicities are likely multifactorial, possibly due to a combination of genetic and environmental factors.4
The timing of infections and/or a dysregulated immune system are postulated to be key factors in ALL etiology.5 In addition, because an individual’s response to infection can be highly variable and ALL is a cancer of lymphoid cells, it has been hypothesized that genetic variants in immune system genes may contribute to ALL etiology. This effect could also be influenced by population-specific genetic variants that control the immune response. Although many studies have been conducted, the results are inconsistent.4,6
Natural killer (NK) cells express killer cell immunoglobulin-like receptors (KIRs), which interact primarily with HLA-C ligands. The KIR gene family located on 19q13.4 consists of 14 genes and 2 pseudogenes encoding receptors that are expressed on NK cells.7 The KIR locus on 10q13.4 has 2 primary haplotypes, A and B; KIR A contains 1 activating gene and KIR B is more polymorphic and contains the other 5 activating genes. The 6 activating KIR genes encode receptors that activate NK-cell activity upon binding of ligands such as HLA-C1, HLA-C2, HLA-Bw4, and HLA-Bw6.
In this issue, de Smith and colleagues performed a case-control study of the association between pediatric ALL and the activating and inhibitory KIR genes, as well as the HLA-C group 1 (C1), group 2 (C2), and HLA-Bw4 polymorphic residues.1 The 212 ALL cases (including 114 Hispanic and 76 non-Hispanic white) in this study were children younger than 15 years of age enrolled in the California Childhood Leukemia Study. The controls (128 Hispanic and 86 non-Hispanic) were derived from the California Department of Public Health’s Genetic Disease Screening Program. This study showed that there was a statistically significant association between the KIR A haplotype (as well as the number of activating or inhibitory KIR genes) and ALL in the Hispanic cases but not in the non-Hispanic cases when compared with ethnically matched controls. The converse was found in the HLA-Bw4/Bw6 genetic variants when the association was present in the non-Hispanic cases but not in the Hispanic cases. There was no association between ALL and HLA-C1 or HLA-C2 in either population.
In 2011, Almalte et al evaluated the 6 stimulating activating KIR genes in a case-control study of 100 B-cell ALL cases and 245 controls of French Canadian ancestry.8 This study found a statistically significant inverse association between the presence of activating KIR genes and childhood ALL. These findings were consistent in a population of 45 non-French ancestry, white Canadian individuals. This study did not evaluate KIR inhibitory genes or haplotypes.
Notably, no association was found between KIR variants and childhood ALL in a case-control study from Germany (92% German ancestry) of 185 B-cell ALL cases. This study included both activating and inhibitory KIR genotypes9 and haplotype analyses. Consistent with the German study,9 the current study of individuals from California did not find an association between KIR polymorphisms and ALL in non-Hispanic whites.1
As authors of all 3 studies point out, the discrepancies in their results could be due, in part, to differential accuracy of the genotyping methodology across the KIR locus, and/or small differences between the genetic background of the populations studied.1,8,9 Although these 3 studies evaluated the KIR locus, they did not use the same genotyping methods or analyze the data in the same manner. Only the 6 activating genes were evaluated in the French Canadian study. Both activating and inhibitory KIR genes, as well as haplotype analyses, were studied in the German and California studies.
It is intriguing to consider that an important component of ALL etiology could be due to population-specific genetic variants. Variants in ARID5B and PIP4K2A loci are differentially associated with ALL risk based on ethnicity.4 The concept of evaluating the underlying population’s genetic structure was successfully applied through the use of mapping by admixture linkage disequibrium in a genome-wide association study (GWAS) of relapse after ALL therapy.10 That study found there were specific ancestry-related genetic differences associated with relapse, even after adjusting for known prognostic factors, that could partially explain the differences in survival between ethnic groups.
In contrast, studies across key immune loci, such as the expanded major histocompatibility complex, have not consistently found differences between ethnicity, genetic variation, and ALL.6 The finding of de Smith et al that HLA-Bw4 was associated with ALL in non-Hispanic whites but not in Hispanic individuals, coupled with the opposite finding in the association between KIR haplotypes and ALL in Hispanics, may provide a clue as a potential connection between these loci and ALL risk in different ethnic groups.
Differences in the immune response as well as environmental exposures could be key components of the disparities seen in both ALL incidence and clinical outcomes between Hispanic and non-Hispanic individuals with ALL. Epidemiology and genetic association studies have yielded important insights into these possible links, but many questions remain, due, in part, to the challenges in studying a relatively rare cancer. Many ALL etiology studies have limited statistical power due to their small sample sizes. GWAS have taught us that the effects of population stratification can be significant in case-control studies utilizing self-identified ethnicities. Future studies of connections between genetic variation in the immune system and ALL would benefit from careful evaluation of the underlying population structure and the creation of genetically matched controls. This approach, coupled with detailed environmental exposure assessments, has the potential to help sort out some of the reasons for inconsistent study results and to greatly advance our understanding of ALL etiology.
Conflict-of-interest disclosure: The author declares no competing financial interests.