Abstract
T cells redirected to specific antigen targets with engineered chimeric antigen receptors (CARs) are emerging as powerful therapies in hematologic malignancies. Various CAR designs, manufacturing processes, and study populations, among other variables, have been tested and reported in over 10 clinical trials. Here, we review and compare the results of the reported clinical trials and discuss the progress and key emerging factors that may play a role in effecting tumor responses. We also discuss the outlook for CAR T-cell therapies, including managing toxicities and expanding the availability of personalized cell therapy as a promising approach to all hematologic malignancies. Many questions remain in the field of CAR T cells directed to hematologic malignancies, but the encouraging response rates pave a wide road for future investigation.
Introduction
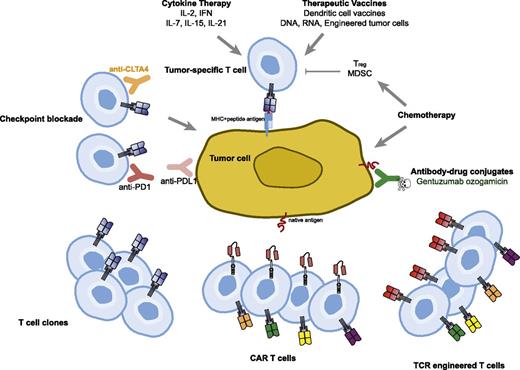
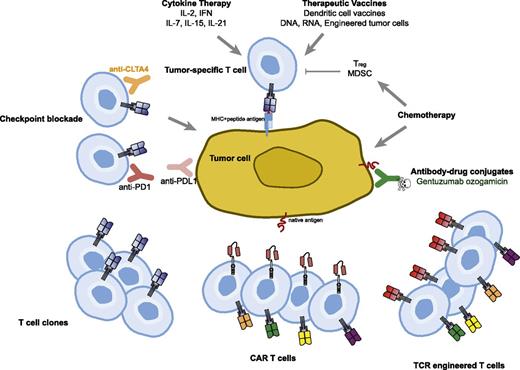
Immune-based therapies for cancer have the tantalizing possibility of effecting long-term durable remissions and perhaps even offering the possibility of a cure. This is the basis for the US Food and Drug administration (FDA) approval of interleukin-2 (IL-2) in melanoma; more recent immune therapies that are FDA-approved treatments for cancer involve checkpoint blockade, which is a form of releasing the brakes on tumor-specific T cells and allowing them to persist and expand in vivo, leading to control or regression of cancer. Adoptive T-cell therapy also offers this possibility but has thus far been limited in application to those patients with melanoma who have adequate culture and expansion of isolated tumor-infiltrating lymphocytes.1 The main barriers to this approach have been the difficulty in culturing and manufacturing of tumor-infiltrating lymphocytes, immune tolerance to self-antigens, and the requirement for major histocompatibility complex (MHC) presentation of antigens (Figure 1).
Therapeutic approaches to overcome immune tolerance to tumors. Cytokines and vaccines can be used to augment natural T-cell responses to tumor. Antibodies targeting negative regulatory molecules such as programmed death 1 (PD-1) and cytotoxic T-cell lymphocyte-associated antigen 4 (CTLA-4) can be infused to release the brakes on natural T cells responsive to tumor. Chemotherapy can reduce immune suppressive cells such as Tregs and myeloid-derived suppressor cells (MDSC) in addition to its direct effect on the tumor cells. Adoptive T-cell transfer strategies using clonally expanded cytotoxic T cells or T cells engineered to express TCRs or CARs are being tested.
Therapeutic approaches to overcome immune tolerance to tumors. Cytokines and vaccines can be used to augment natural T-cell responses to tumor. Antibodies targeting negative regulatory molecules such as programmed death 1 (PD-1) and cytotoxic T-cell lymphocyte-associated antigen 4 (CTLA-4) can be infused to release the brakes on natural T cells responsive to tumor. Chemotherapy can reduce immune suppressive cells such as Tregs and myeloid-derived suppressor cells (MDSC) in addition to its direct effect on the tumor cells. Adoptive T-cell transfer strategies using clonally expanded cytotoxic T cells or T cells engineered to express TCRs or CARs are being tested.
The infusion of gene-modified T cells directed to specific target antigens offers the same possibilities of long-term disease control and has the added benefit of the rapid onset of action that is usually seen with cytotoxic chemotherapy or with targeted therapies. In particular, T cells modified to express antibody-based chimeric antigen receptors circumvent both immune tolerance of the T-cell repertoire and MHC restriction. Furthermore, advances in the culture process and molecular and virology techniques used to introduce novel genes into T cells have made the manufacturing of gene-modified peripheral blood–derived T cells relatively straightforward.
In the last 5 years, chimeric antigen receptor (CAR)-redirected T cells have emerged from the bench and made splashy headlines in the clinical setting at a number of academic institutions. It is not surprising that CAR T cells directed to hematologic malignancies have been the first ones tested, given the extent of the known surface antigens expressed on hematologic cells, the relative ease of sampling tumor, and the natural preference of T-cell homing to hematologic organs such as the blood, bone marrow, and lymph nodes. Here, we will introduce the various CAR designs that have been tested clinically, the results from a series of clinical trials testing CAR T cells, and an overview and comparison of the manufacturing processes used. We will also discuss the emerging toxicity profiles and management strategies and future outlook of CAR T-cell therapies. We limit our discussion to CAR T cells in hematologic malignancies and will not cover CARs that have been tested in solid tumors or engineered T-cell receptors (TCRs) that have been tested in any setting.
Anatomy of CARs and CAR T-cell products
CARs are synthetic, engineered receptors that can target surface molecules in their native conformation.2 Unlike TCRs, CARs engage molecular structures independent of antigen processing by the target cell and independent of MHC. CARs typically engage the target via a single-chain variable fragment (scFv) derived from an antibody, although natural ligands have also been used.3 Individual scFvs targeting a surface molecule are either derived from murine or humanized antibodies or synthesized and screened via phage display libraries.4 Unlike TCRs, where a narrow range of affinity dictates the activation and specificity of the T cell, CARs typically have a much higher and perhaps broader range of affinities that will engage the target without necessarily encountering cross-reactivity issues. Preclinical data suggest that the spatial location of epitope binding has a bigger effect on CAR activity than variation in affinity.5 The length, flexibility, and origin of the hinge domain is also an important variable in the design of CARs.6-8 A major challenge to the field is that it is currently necessary to empirically test these design variables as there are no general rules guiding CAR design for target molecules.
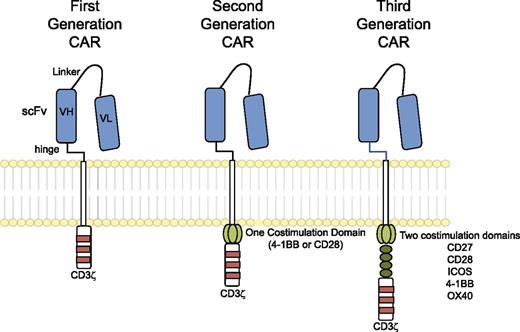
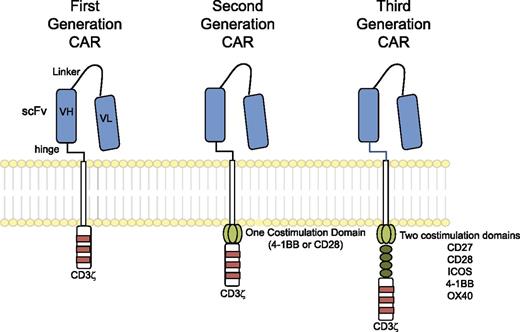
The “generations” of CARs typically refer to the intracellular signaling domains. First-generation CARs include only CD3ζ as an intracellular signaling domain, whereas second-generation CARs include a single costimulatory domain derived from either CD28 or 4-1BB; third-generation CARs include two costimulatory domains, such as CD28, 4-1BB, and other costimulatory molecules (Figure 2). The hinge and transmembrane domains are probably the least commented on aspect of CAR design, though they may make important contributions to the interaction with antigen, assembly of the immunologic synapse, and association of the CAR with other proteins necessary to transduce a robust activation signal. Most investigators are using the hinge and transmembrane domains of CD8α or CD28; hinge domains derived from Fc regions have also been investigated and modified in length6 and have also been reported to engage Fc receptors and activate innate immune cells.7,8
Chimeric antigen receptors. CARs target surface antigens in an MHC-independent fashion and consist of an ectodomain, hinge domain, transmembrane domain, and endodomain. The initial trials tested first-generation CARs that have a single cytoplasmic domain. Current trials are testing second- and third-generation CARs that have combinations of signaling domains.
Chimeric antigen receptors. CARs target surface antigens in an MHC-independent fashion and consist of an ectodomain, hinge domain, transmembrane domain, and endodomain. The initial trials tested first-generation CARs that have a single cytoplasmic domain. Current trials are testing second- and third-generation CARs that have combinations of signaling domains.
Investigators in the field are using a variety of methods to introduce their CAR constructs into T cells.9 Each has advantages and disadvantages with regard to cost, safety, and level of expression. Non–viral-based DNA transfection was initially used because of cost and the low risk of insertional mutagenesis. This method requires long-term culture and antibiotic selection due to the relative inefficiency of gene transfer. The long-term culture may be detrimental to the activity and persistence of the infused cells, and the antibiotic-resistance gene products may render them immunogenic. Transposon-based systems can integrate transgenes more efficiently than plasmids that do not contain an integrating element.10,11 Sleeping Beauty was shown to provide efficient stable gene transfer and sustained transgene expression in multiple cell types, including T cells,12 but the culture time remains relatively prolonged. This system13 is now entering clinical trials as an approach to engineer T cells.
Most investigators have been using γ retroviruses; these are relatively easy to produce, they efficiently and permanently transduce T cells, and they have preliminarily proven safe from an integration standpoint in primary human T cells.14 One potential disadvantage of retrovirus as the vehicle is the potential for silencing of expression of the CAR based on silencing of the long terminal repeats; in the right context, this could be an advantage of CAR-based therapies if they are to be used as a bridge to another definitive treatment such as allogeneic bone marrow transplant. Lentiviral vectors also efficiently and permanently transduce T cells but are more expensive to manufacture; they are also potentially safer than retrovirus based on integration preferences15 examined in hematopoietic stem cells, though it is not clear that this applies to primary human T cells. Use of specific promoters in combination with lentiviral transduction has enabled sustained surface expression of CARs on transduced T cells; this likely extends the survival of functional CAR T cells in vivo.
The methods and duration of T-cell culture used in the manufacturing of CAR T cells may also be an important variable in the composition of the final CAR T-cell product. Generally, the options have involved combinations of TCR stimulation through antibodies and supportive cytokines or artificial antigen-presenting cells that are either cell based or bead based.16 Culture conditions that combine costimulation and various cytokines support the maintenance of a central memory phenotype.17,18
Review of clinical data with CAR T cells in hematologic malignancies
There are 14 publications reporting clinical trials of CAR T cells in hematologic malignancies. All but one of these focused on B-cell malignancies by targeting CD19 or CD20; the other focused on acute myeloid leukemia (AML) by targeting Lewis-Y antigen. The CAR design, manufacturing process, and results are summarized in Table 1.
Each group has designed slightly different protocols, and they vary with regard to design of the CAR, expression of the CAR on the T cells, T-cell culture conditions, lymphodepleting strategy, cytokine support for the infused T cells, disease targeted, and timing of CAR T-cell infusion with regard to standard therapy such as bone marrow transplantation. Although the number of variables across trials makes for challenging comparisons, together the clinical data suggest that the most effective CAR T cells exhibit high levels of CAR expression before infusion and expand and persist in vivo, ie, “engraft,” for at least several weeks. Not surprisingly, the most effective CAR T-cell products are also associated with on-target toxicity. The details of trials conducted at the Fred Hutchinson Cancer Research Center, City of Hope, Baylor College of Medicine, MD Anderson Cancer Center, National Cancer Institute, Memorial Sloan-Kettering Cancer Center (MSKCC), the University of Pennsylvania, and the University of Melbourne are found in supplemental “Materials”.19-41
Lessons learned from clinical experience
The results of these clinical trials point to several key factors that may have an impact on the efficacy of CAR-modified T cells in hematologic malignancies. One is that the disease under study may be differentially susceptible to CAR-mediated T-cell killing; for example, although all express CD19, it appears that acute lymphocytic leukemia (ALL) has a higher response rate than chronic lymphocytic leukemia (CLL) or indolent lymphomas, with an impressive (∼80%) response rate across CAR designs, trial designs, and institutions. This could be true for several reasons, including host T-cell defects in lymphomas such as those described in CLL patients,42,43 inhibitory effects of the tumor microenvironment,44,45 the length and nature of prior treatments, the age of the patient, and the robustness and composition of the T cells in the starting product and in the infused product. At the level of product characterization, the relative importance of the CD4:CD8 ratio or the proportion of regulatory T cells (Tregs) in the final product is not clear, and it is not known if the T-cell product can be improved upon by selection or graft engineering. Characterization of the tumor microenvironment, and in particular the inhibitory factors that may affect or abrogate CAR T-cell lytic functions, will be even more complex to sort out. Both gene expression profiling and flow cytometric analysis of recovered T cells postinfusion show that infused CAR T cells express PD134,46 and are susceptible to PD1/PD-L1 interactions. Encouragingly, based on preclinical animal modeling,47 checkpoint blockade is already being tested in clinical trials in combination with CAR T cells. Several investigators have also aimed to address the question of whether or which lymphodepletion strategy to use and whether supporting the infused T cells with systemically administered cytokines will improve their expansion or persistence; although both strategies appear to be improve T-cell engraftment, they may confound interpretation of efficacy and toxicity, and neither appears to be universally required for CAR T-cell–mediated responses.
At least a few key characteristics of efficacious CAR T-cell products have emerged. One is that expression of the CAR at the cell surface seems to be required for efficacy; another is that in vivo detection of the CAR T-cell product in the blood is a sign of adequate engraftment and that engraftment is required for responses. Detection of the CAR transgene by polymerase chain reaction does not inform about the surface expression of the CAR, which is the only form that matters for efficacy. Thus, the availability of reagents to specifically detect CARs at the cell surface by flow cytometry is crucial to understand the activity and engraftment of CAR T cells. In addition, it is not yet clear if there is a relationship between the dose of CAR T cells administered and the level of engraftment that is achieved, ie, the in vivo dose. When CAR T cells expand efficiently, very low doses of CAR T cells can still exert dramatic effects and control tumor33 ; given the complexity of manufacturing CAR T cells, the ability to administer low doses of an effective product is very appealing.
Some degree of persistent engraftment is also required, although the length of this persistence has not been established. We hypothesize that for CAR T cells to be able to replace allogeneic transplantation as definitive therapy, persistence for at least several months will probably be required based on the kinetics of tumor clearance that we have observed.37 If, on the other hand, CAR T cells are only to serve as a bridge to follow on therapy with allogeneic transplant, then they may only need to persist for a few weeks until conditioning for the transplant begins. The question of whether CAR T cells are on par with the efficacy of transplant would best be answered in randomized trials. However, short of this, we also anticipate many recipients of CAR T cells will not eligible for transplant or have comorbidities or only suboptimal donors available such that the transplant is impractical. Long-term follow up of these patients will provide critical information about the durability of CAR T-cell–induced remissions. Alternatively, the answer may come from patients who have relapsed after an allogeneic transplant, for whom a second transplant may not be possible or efficacious; these patients may benefit from CAR T-cell products that potentially offer durable remissions. The use of CAR-modified donor T cells to treat relapsed ALL is being tested by our group and will hopefully be studied in a multisite trial. In addition, multisite trials are underway in collaboration with Novartis, and a dual-center grant-funded trial between MSKCC and the University of Pennsylvania involves a competitive repopulation study design where T cells transduced with Penn vector and MSKCC vector are infused simultaneously into each patient.
Toxicities and management: from CRS, CNS, and MAS/hemophagocytic lymphohistiocytosis to B-cell aplasia
Given the finding that, in most cases, cytokine release syndrome (CRS) seems to correlate with antitumor activity, one question that has emerged is the degree to which the innate immune system contributes to antitumor efficacy. In addition, we have shown that CRS is often accompanied by a macrophage activation syndrome (MAS), which may be driven in part by high levels of IL-6.37 Although it is straightforward to hypothesize that CAR T cells directly kill tumor cells, it is not entirely clear which cell type produces the vast majority of the cytokines, particularly IL-6 (which our work has demonstrated may be key to the toxicity response),37,51 and whether blockade of cytokines with anticytokine therapy such as tocilizumab or general immune suppression with corticosteroids affects the antitumor response. It is possible that the IL-6 is produced by the dying B cells, dying tumor cells, or activated macrophages that are recruited to digest lysed tumor cells.
Does interruption of the cytokine cascade lead to interruption of the antitumor effect? This remains an unanswered question and has direct clinical impact for patients and physicians deciding on when to abort the CRS. Furthermore, although, in our experience, most responding patients have some degree of CRS, it is not yet clear whether the severity of CRS or macrophage activation syndrome (MAS) is related to antitumor efficacy. The severity of CRS does appear to be related to the tumor burden. If engagement of the innate immune system contributes to the mechanism of action, this could bode well for the use of CAR T cells in solid tumors, where T cells may not preferentially home to and persist at the sites of tumors as efficiently as they do in hematologic malignancies.
Several patients in CD19-CAR trials across institutions have experienced obtundation, seizures, aphasia, and mental status changes, which have all been reversible. Some of these may be related to CRS, but whether this results from systemic cytokines crossing the blood-brain barrier and engaging cytokine receptors in the brain or from direct cytokine production in the central nervous system (CNS) is not clear. Many of these patients develop MAS, and MAS is often associated with neurologic toxicity.38-40 In addition, we have unexpectedly found CAR T cells in the cerebrospinal fluid of asymptomatic patients, even when there is no evidence of CD19+ disease there. It is possible that the hyperthermia and IL-6 release during CRS enhances trafficking of CAR T cells to the cerebrospinal fluid in an antigen-independent mechanism.48 It is also possible that there is some cross-reactivity or as-yet-undetected expression of CD19 in the brain. Blinatumomab, a type of bispecific T-cell–engaging antibody (BiTE) that is a fusion protein between an anti-CD19 scFv and an anti-CD3 scFv, also has neurologic toxicity and seizures as its dose-limiting toxicity, even though it does not appear to control CNS disease. It is interesting that blinatumomab has also been shown to cause MAS.49 Optimistically, CAR T cells may provide a way of controlling occult or frank CNS malignancy without chemotherapy or radiotherapy.
B-cell aplasia is an expected on-target result of CD19-directed therapies and has served as useful surrogate to determine the persistence and effectiveness of CD19-directed CAR T cells. Fortunately, B-cell aplasia is a manageable disorder; patients may be infused with γ-globulin as replacement therapy, though this could become an expensive and difficult treatment to implement across all diseases that may be eventually treated with CAR T cells. Persistent B-cell aplasia could also result in an increased risk of infection even with replacement therapy. In an ideal setting, the CAR T cells would persist long enough to mediate definitive control of disease but then allow for recovery of normal B-cell and plasma cell recovery such that patients could be revaccinated.
As more patients are treated with CAR T cells directed to CD19, clinician investigators will need to establish straightforward algorithms for management of toxicities, including the optimal timing and dose of administration of cytokine blockade, corticosteroids, and immunoglobulin replacement.
Because gene-modified T cells are emerging as powerful therapies capable of effecting dramatic antitumor responses as well as significant toxicities,50 strategies to incorporate suicide genes or abortive mechanisms may become necessary. This is especially true as CAR-directed T cells are further engineered to express cytokines and adjuvants51,52 that could act in trans to amplify inflammatory cascades, or as CAR T cells directed to antigens with more widespread expression are tested. However, suicide systems are still difficult to implement in all CAR T-cell trials, because many of the suicide systems are immunogenic (eg, herpes simplex virus thymidine kinase) or require intravenous administration of the suicide-inducing prodrug.53 Alternatively, altering the homing of T cells via enforced expression of chemokine receptors54 or pharmacologic blockade of chemokine receptors55 may be a strategy to both enhance efficacy and alleviate toxicity.
Future outlook
Manufacturing
All investigators involved in CAR T-cell trials are acutely aware of the technical, regulatory, and financial challenges in manufacturing single-patient product lots. One potential solution is to generate universal T-cell products from allogeneic donors, based on knockdown of the HLA genes coupled with enforced expression of nonclassical HLA molecules to avoid natural killer (NK) cell–mediated recognition and lysis.56,57 In addition, it may not be necessary to use T cells with integrated CAR transgenes, because transient expression of CARs with RNA transfection has also been effective in preclinical models.58 Similarly, because the cumulative experience with retroviral or lentiviral transduced T cells demonstrates a notable lack of generation of replication-competent retroviruses,59,60 it is possible that the testing requirements for this will be relaxed, which would make manufacturing less expensive and shorten the time from completion of culture to potential infusion, which is an important consideration in patients with aggressive malignancies.60 As CAR T cells enter the mainstream of the therapeutic armamentarium for hematologic malignancies, more streamlined and centralized manufacturing will need to be established, with shorter times in culture.61 In addition, the use of serum-free medium will be mandatory, because projections indicate an insufficient world supply that will peak in a few years, a situation similar to the concept of “peak oil” production.62
Defining the “active ingredient” and cell type
Although CAR T cells have been developed entirely in the academic setting, the recent FDA publication of a Draft Guidance for Industry on early phase trials of cell and gene therapies63 indicates a shift of this concept from a fringe research topic to acceptance as a mainstream investigational product. One mandate of the guidance is for sponsors to attempt to define the active ingredient in the cell or gene therapy product. For gene-modified T cells, there are multiple factors that contribute to the definition of the active ingredient: optimal vector, culture conditions, CAR design, cell type, and dose of that cell type. The simplest way to define the active ingredient at the moment is the number of CAR+ cells. However, the precise type of cell that is transduced may be important in defining the active ingredient as well. For example, it may be that only the central memory CD8+ T cells contribute to engraftment and therefore are the only cell type that contributes to the active ingredient.
Most investigators have focused on peripheral blood–derived T cells and subsets of these such as virus-specific T cells, central memory T cells, or cord blood–derived T cells.22 However, there is also potential for the use of multilineage effector cells when hematopoietic stem cells or other precursor cells are used as the starting cell type.64-66 Defining the phenotype or active ingredient of these cell types will be even more challenging than T cells. Finally, NK cells can also be powerful effector cells, and some investigators have transduced NK cells with second-generation CARs.67 Although technically outside the scope of this review, “BiKEs” (bispecific killer engagers) engage NK cells with bispecific antibodies to the target antigen (CD33) and the NK activator CD1668 ; these are a corollary to BiTEs, which are bispecific engagers of T cells, like blinatumomab; both of these engage the immune system with an antibody-like structure.
New targets
Aside from defining the optimal cell product, there are 2 main hurdles in broadening the use of CAR-directed T cells beyond B-cell malignancies: target discovery and manufacturing on a wide scale. Several investigators are investigating new targets for myeloma, including BCMA,69 CD70,70 CD74,71 CD38, CD138, and CS1.67 Some of these targets are purely based on differential expression of the target in myeloma plasma cells, whereas others are based on encouraging results with either antibody-drug conjugates or naked antibodies such as CS1-elotuzumab72 and CD38-daratumomab.73
In AML, there are currently no open trials of CAR-modified T cells in the United States, though there are trials open in China and in Australia targeting CD33 and Lewis-Y, respectively (Table 2). Based on the clinical experience with the anti-CD33 drug conjugate gemtuzumab ozogamicin (Mylotarg), a CD33-directed CAR may also be evaluated, and preclinical data with a CD33/CD3 BiTE is encouraging.74 Preclinical data of CD123-directed CARs have been reported by 2 groups.75,76 A CD44v6 CAR has also been tested in in vitro and in xenogeneic models of AML and myeloma77 ; these investigators found that in vitro activation with anti-CD3/28 beads and culture in IL-7 and IL-15 were necessary for antitumor efficacy in vivo.
Baylor has 2 open clinical trials targeting CD30 in Hodgkin disease, and the University of Cologne plans to treat patients with mycosis fungoides with CD30 CAR T cells encoding a CD28 domain with a deleted lck binding moiety, based on preclinical data that this reduces Tregs in the tumor microenvironment.78 There are also open trials targeting the immunoglobulin G κ light chain in B-cell malignancies including lymphoma, myeloma, and leukemia; attractive aspects of this approach are the suitability of targeting myeloma and that theoretically, on-target toxicity will be a more limited B-cell aplasia than what is seen with CD19 CARs that target all B cells. Finally, although no CD19-negative escape variants of CLL have been described, other potential targets for CLL are CD23 and R0R1, and preclinical data are promising.79,80
Conclusions
In the case of CD19-directed CAR T cells, multisite trials are in the planning stages for several groups and now include involvement of the pharmaceutical and biotechnology industries as well as cooperative group networks. This is an exciting time in the treatment strategies for all hematologic malignancies; a decade ago, few would have predicted that the promises of gene-modified cell therapies would be delivered by CAR T cells directed to aggressive hematologic malignancies such as adult and pediatric B-cell ALL.
Authorship
Contribution: M.V.M. and C.H.J. wrote the manuscript, and S.A.G. and D.L.P. edited the manuscript.
Conflict-of-interest disclosure: M.V.M., D.L.P., and C.H.J. have patents in the field of adoptive immunotherapy. All authors have sponsored research grant support from Novartis.
Correspondence: Marcela Maus, 3400 Civic Center Blvd, 8th Floor, Philadelphia, PA, 19104-5156; e-mail: marcela.maus@uphs.upenn.edu; and Carl June, 3400 Civic Center Blvd, 8th Floor, Philadelphia, PA, 19104-5156; e-mail: cjune@exchange.upenn.edu.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Anne Chew and Bruce Levine for constructive comments.
This work was supported in part by grants from the National Institutes of Health, National Cancer Institute (K08 CA166039, 5R01 CA165206, and R01 CA120409), the Pennsylvania Department of Health, Cookies for Kids Cancer, and the American Cancer and Leukemia and Lymphoma Societies (7000-02).