To the editor:
Coagulation abnormalities are frequent in systemic light-chain (AL) amyloidosis,1 6.3% to 14% present with acquired factor X (FX) deficiency reported,1-3 with baseline FX levels not predictive of bleeding risk.4 Rapid clearance of 131I-labeled FX from the circulation to areas of amyloid deposition, particularly within the spleen,5,6 suggests adsorption on amyloid fibrils as a major cause of FX deficiency in AL. Other proposed explanations include synthetic dysfunction caused by liver involvement or vitamin K deficiency and discordance between FX activity and FX antigen.1 Until recently, treatment of FX deficiency was limited to therapeutic options including fresh frozen plasma (FFP), prothrombin complex concentrates (PCCs), activated PCCs (aPCC), or recombinant factor VIIa (rFVIIa)7 —each with significant risks in this fragile patient population. Forty-four percent of patients in a larger series had complications when treated with rFVIIa preoperatively, including bleeding, thrombosis, or death.4 In addition to FFP, PCC, aPCC, or rFVIIa, tranexamic acid, plasma exchange,8 and splenectomy3 were anecdotally reported as treatments for FX deficiency in AL, the latter aimed at removing the splenic amyloid fibril burden. Because of a variable amount of FX in FFP, large volumes are needed to achieve a hemostatic effect with risk of fluid overload in patients with cardiac involvement. A serious concern arises regarding thrombotic risks of FVIIa or aPCC in patients with AL amyloidosis who are elderly with cardiovascular risk factors and often nephrotic as a result of renal involvement (hence inherently prothrombotic).
BPL (Bio Products Laboratory Ltd., Elstree, United Kingdom) have developed a high-purity plasma–derived FX concentrate which is currently undergoing phase 3 clinical trials for patients with hereditary FX deficiency. One patient with inherited FX deficiency presenting with a shoulder hemarthrosis achieved good hemostatic response after FX dose of 25 IU/kg per day9 with a biological half-life of 24 to 48 hours.
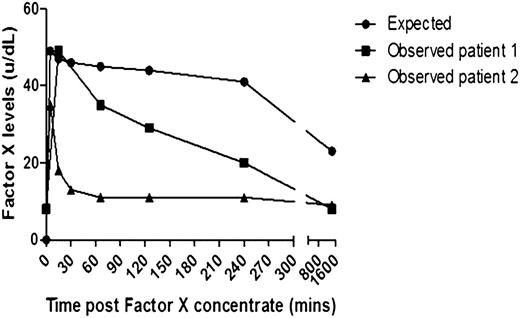
This is an initial report of using high purity FX (HP-FX) concentrate in 2 patients with systemic AL amyloidosis and acquired FX deficiency. Two patients with biopsy-proven AL amyloidosis, with evidence of liver involvement, and a large amyloid load on 123I serum amyloid P component scintigraphy, presented with a forearm hematoma (patient 1) and a ruptured spleen and knee hemarthrosis (patient 2) caused by acquired FX deficiency (both with FX levels of 8 U/dL), with patient characteristics illustrated in the supplemental Table (available on the Blood Web site). The bleeding episode in each case was treated with HP-FX concentrate given as a single dose of 40 IU/kg (FX activity assessed by one-stage clotting prothrombin time–based assays) and hemostasis achieved in conjunction with supportive measures. There was a striking difference between FX recovery reported in patients with inherited FX deficiency compared with our AL patients, the latter showing less predictable kinetics and a more rapid decline to baseline in 2 to 4 hours (Figure 1), consistent with the theory of FX adsorption on amyloid fibrils. Each patient with AL amyloidosis has a different amyloid load and a unique fibril sequence with a potentially different avidity for binding FX—both likely to lead to large differences in the half-life and dose needed, as in patient 2, with a larger load and earlier decrease in FX levels.
The graph demonstrates FX levels post infusion of a single dose of 40 IU/kg of high-purity FX concentrate in 2 patients with AL amyloidosis with acquired FX deficiency. Patient 1 (line with squares) and patient 2 (line with triangles) compared with a reference line constructed from FX levels of 3 patients with hereditary FX deficiency reported by Alvarez et al9 given the same concentrate at a dose of 25 IU/kg (line with circles).
The graph demonstrates FX levels post infusion of a single dose of 40 IU/kg of high-purity FX concentrate in 2 patients with AL amyloidosis with acquired FX deficiency. Patient 1 (line with squares) and patient 2 (line with triangles) compared with a reference line constructed from FX levels of 3 patients with hereditary FX deficiency reported by Alvarez et al9 given the same concentrate at a dose of 25 IU/kg (line with circles).
In summary, high-purity FX concentrate is useful in treating bleeding caused by acquired FX deficiency in systemic AL amyloidosis. Higher and/or more frequent dosing is likely to be required to achieve adequate FX levels for hemostasis with frequent monitoring of FX levels given the unpredictable kinetics; target FX thresholds similar to patients with inherited FX deficiency: 10 to 15 IU/dL for minor bleeding and >50 IU/dL for major bleeding, trauma, or surgery. HP-FX has the advantage that the hemostatic response can be monitored and the treatment tailored to the patient’s individual needs.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors gratefully acknowledge BPL for providing high purity FX concentrate for treatment of these patients on a named-patient basis.
Contribution: S.M. and A.D.W. designed the study, analyzed data, and wrote manuscript; J.B., A.D., and P.N.H. treated patients and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashutosh Wechalekar, National Amyloidosis Centre, UCL Medical School (Royal Free Campus), Rowland Hill St, London, NW3 2PF, United Kingdom; e-mail: a.wechalekar@ucl.ac.uk.