In this issue of Blood, Middeke and colleagues highlight the poor outcome of the abnormal (17p) [abnl(17p)] subgroup of cytogenetically adverse-risk acute myeloid leukemia (AML) even after allogeneic hematopoietic stem cell transplantation (HSCT).1
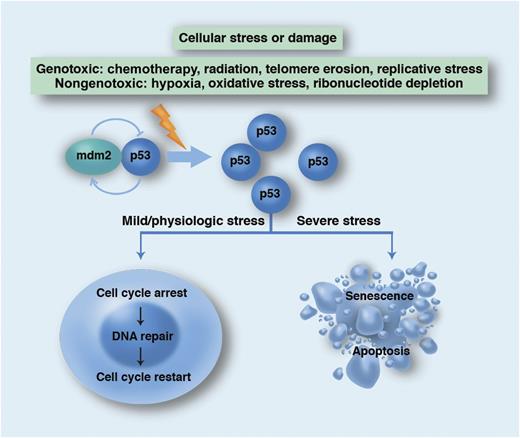
Schema for the physiological role of the p53 network in promoting genetic stability. WT p53 is normally targeted for proteasomal degradation by mdm2 but accumulates in states of cellular stress or damage. p53 transcriptionally regulates numerous target genes (including p21) as well as interacts directly with other proteins to either quarantine the cell for repair or condemn it to die. Professional illustration by Marie Dauenheimer.
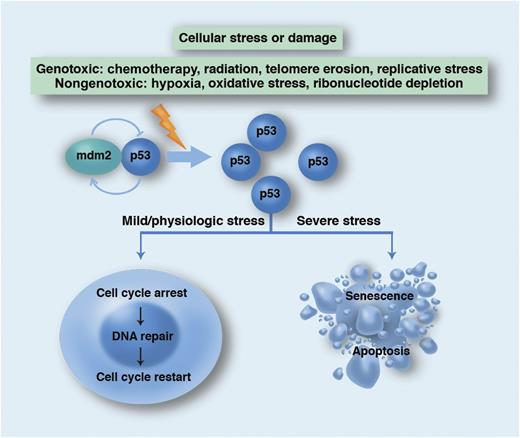
Schema for the physiological role of the p53 network in promoting genetic stability. WT p53 is normally targeted for proteasomal degradation by mdm2 but accumulates in states of cellular stress or damage. p53 transcriptionally regulates numerous target genes (including p21) as well as interacts directly with other proteins to either quarantine the cell for repair or condemn it to die. Professional illustration by Marie Dauenheimer.
Metaphase cytogenetics at the diagnosis of AML is routinely used to establish favorable, intermediate, and unfavorable prognostic categories. Unfavorable-risk cytogenetics is a standard indication for HSCT in first complete remission (CR1) for subjects with an available donor. Further dissection of the impact of individual cytogenetic abnormalities within the unfavorable-risk group is ongoing by retrospective analyses of registry studies. Eventually, it will be possible to identify patients who would unequivocally benefit from HSCT vs those who would be better served by participating in clinical trials with newer antileukemic therapies.
Abnl(17p) has emerged as one of the worst cytogenetic aberrations in AML. Although abnl(17p) may be conveniently identified by conventional metaphase cytogenetics or fluorescence in situ hybridization (FISH), it is only a part of the mutational spectrum in the tumor protein 53 (TP53) gene. TP53 is highly conserved across vertebrates and, in humans, is located on chromosome 17p. Most mutations in TP53 remain cryptic and are only identified by gene sequencing. What do we know about TP53 mutations in AML? Whereas cytogenetically defined abnl(17p) is seen in ∼5% of AML, the frequency of TP53 mutations found by sequencing is 8% in de novo AML.2 TP53 mutation is the hallmark genetic aberration, rising to ∼30%, in secondary AML.3 Significantly, TP53 mutations are driver mutations, in most cases mutually exclusive with mutations involving the transcription factor fusion genes NPM1, RUNX1, Flt3-ITD, or CEBPA and are highly correlated with a complex karyotype.2,4-6
In this retrospective analysis of pooled registry data, Middeke et al have reported on the outcomes of 201 AML subjects with abnl(17p) by cytogenetics or FISH, all of whom underwent allogeneic HSCT. This is the first large retrospective study to derive definitive conclusions about the value of HSCT with this cytogenetic abnormality. Overall survival (OS) was only 22% even in CR1, and early relapse was the greatest contributor to mortality with a cumulative incidence estimate of 49%.1 Such dismal outcomes are comparable to the monosomal karyotpye7 and Flt-3 ITD8 mutations that confer OS of ∼20% despite prompt HSCT in CR1. Although the OS with abnl(17p) after HSCT is certainly better than the OS of 0% described in another large series,4 it is clear that HSCT produces disappointing outcomes.
The poor prognosis of this subtype of AML is not at all surprising. TP53 is frequently referred to as the “guardian of the genome” based upon its crucial role as a tumor suppressor gene in protecting against mutations in the genome. TP53 mutations may occur infrequently in the germline (Li-Fraumeni syndrome) but more commonly as acquired somatic mutations that are involved in ∼50% of all malignant conditions.9 More than 1000 different somatic mutations have been described, defying drug development. Mutational effects range from recessive loss of function (with an intact functional copy of the wild-type [WT] allele) to dominant-negative mutations (which dimerize to inactivate WT TP53). TP53 works as the critical mediator of a network that senses cellular stress and, in turn, regulates many other genes. TP53 activation either halts cell proliferation in order to facilitate DNA repair or kills the cell when damage is irreparable (see figure). Loss of TP53 function has been associated with invasion, proliferation, and metastasis. Importantly, TP53 mutation also confers resistance to genotoxic agents, which translates into refractoriness to cytotoxic chemotherapy and irradiation. Conventional small-molecule approaches to restore the functional loss of a defective tumor suppressor have not been successful but there are several promising therapies in development.10 It is therefore notable that the modest benefit of HSCT in abnl(17p) AML probably suggests that there is residual susceptibility to the graft-versus-leukemia (GVL) effect. Definitive evidence for GVL against abnl(17p) AML, such as responses to donor lymphocyte infusions, is still lacking. However, this does support exploring immunotherapeutic approaches against what is currently an “undruggable” target. Given the critical importance of TP53 mutation in oncogenesis and the absence of a US Food and Drug Administration (FDA)–approved therapeutic, there is an urgent need for strategies that may restore the “guardian of the genome” to its guard post.
Conflict-of-interest disclosure: The author declares no competing financial interests.