Key Points
Patients with abnl(17p) AML have a poor outcome after allogeneic hematopoietic stem cell transplantation.
Abstract
Patients with acute myeloid leukemia (AML) and abnormalities of chromosome 17p (abnl(17p)) are at high-risk of treatment failure. Poor outcomes have been reported with conventional chemotherapy. To accurately define the outcome after allogeneic hematopoietic stem cell transplantation (HSCT) in patients with abnl(17p) AML, we analyzed the results of patients with this abnormality who received an allogeneic HSCT between January 2000 and December 2010 in 1 of 4 well-defined cohorts (Fred Hutchinson Cancer Research Center, Haemato Oncology Foundation for Adults in the Netherlands, Study Alliance Leukemia, German Cooperative Transplant Study Group). Data of 201 patients with a median age of 54 years were evaluable. At the time of analysis, 30 patients were alive with a median follow-up of 30 months. The 3-year probability of overall survival (OS) was 15% (95% confidence interval [CI], 10-20). The cumulative incidence of relapse at 3 years was 49% (95% CI, 42-56). Notably, almost 70% of all relapses occurred within the first 6 months after HSCT. Patients who were transplanted in first complete remission (CR1) had superior OS compared with those with advanced disease (22% vs 9%, P < .001). Our findings confirm the high-risk of treatment failure in abnl(17p) AML even after allogeneic HSCT in CR1. Although allogeneic HSCT remains a valid option in CR1, alternative treatment strategies are needed for the remaining patients.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is considered the standard of care as consolidation therapy in patients with high-risk acute myeloid leukemia (AML) as defined by cytogenetic abnormalities.1 This recommendation is mainly based on donor vs no donor analyses, which showed improved disease-free and overall survival (OS) after allogeneic HSCT as compared with autologous HSCT or conventional chemotherapy as postremission therapy.2-6 The improvement in survival after allogeneic HSCT is due to a significantly lower incidence of relapse, presumably caused by graft-versus-leukemia reactions, which is only partly counterbalanced by higher nonrelapse mortality (NRM). For patients with high-risk AML in first complete remission (CR1), the survival benefit with allogeneic HSCT has recently been confirmed in a systematic review and meta-analysis of prospective studies that included 3638 patients.7
Whether this holds true for all cytogenetic entities comprised in the high-risk category remains unclear because subgroup analyses for distinct genetic high-risk abnormalities were not performed in the prospective studies. Recent studies suggest that, within this heterogeneous AML subset, genetic subgroups with a markedly different outcome can be identified.8-10 For example, 1 specific pattern of karyotype abnormalities, the monosomal karyotype (MK), has been shown to identify patients with an extremely poor prognosis both after conventional chemotherapy, but also allogeneic HSCT.6,11-13
Recently, the Study Alliance Leukemia (SAL) retrospectively evaluated the impact of HSCT in patients with abnormalities of chromosome 17p (abnl(17p)) AML who were enrolled into 3 prospective, randomized trials. One hundred and forty-three patients of 3530 patients presented with a newly diagnosed abnl(17p) AML. Forty-seven patients underwent allogeneic HSCT. Multivariate analysis showed no substantial benefit of HSCT on OS (hazard ratio = 0.97; 95% confidence interval [CI], 0.56-1.67]).14 Consistent with this, other groups reported poor survival data after allogeneic HSCT in small numbers of patients with abnl(17p) or TP53-mutated AML.9,10,12
Although the incidence of abnl(17p) diagnosed by conventional cytogenetics is relatively low even in the high-risk group, it increases when incorporating fluorescence in situ hybridization (FISH) analysis or TP53 mutation analysis.15 In a large retrospective repository-based analysis published by Grossmann et al, patients with TP53-mutated AML had the worst prognosis, with a median OS of 4.6 months and event-free survival (EFS) of 0% at 3 years.16 The impact of HSCT was not addressed in that study.
The number of transplanted patients with abnl(17p) included in the studies mentioned was limited, making it difficult to accurately define the outcome of allogeneic HSCT, especially for patients in CR1. Therefore, the aim of this intergroup analysis was to evaluate the results of patients with abnl(17p) AML in a larger cohort and to analyze the impact of different treatment characteristics. Here, we present data of 201 patients with abnl(17p) AML treated with allogeneic HSCT within the past decade.
Patients and methods
Patient population
We performed a retrospective cohort analysis based on study-registries from 2 AML Study Groups, Haemato Oncology Foundation for Adults in the Netherlands (HOVON) and SAL, and Transplant-Registries of the Fred Hutchinson Cancer Research Center (FHCRC) and the German Cooperative Transplant Study Group (GCTSG). Inclusion criteria were AML diagnosed according to the current World Health Organization criteria with cytogenetic abnormalities affecting the critical region 17p13, where the tumor suppressor gene TP53 is located, and first allogeneic transplantation between January 1, 2000 and January 1, 2011. Full karyotype information was required either based on FISH or conventional G-banding techniques. Patients with additional good risk abnormalities were excluded. Patients from the HOVON and SAL study groups were treated within different clinical trials during the given period. The FHCRC and the GCTSG cohorts included all patients transplanted at these centers meeting the inclusion criteria. Double registration of patients was checked. Approval was obtained from the Ethical Review Board of the Technische Universität Dresden for this study. Informed consent was provided according to the Declaration of Helsinki.
Cytogenetic classification
The cytogenetic results were classified independently by 2 scientists according to the European Leukemia Net, MK as introduced by Breems et al,11 and complex karyotype (CK) defined here as 3 or more independent cytogenetic abnormalities.1 Within the CK, we subclassified patients based on the number of aberrations. We also categorized for distinct lesions: additional material of undefined origin replacing part of chromosome 17p [add(17p)], isochromosome for the entire long arm of 1 chromosome 17, deletion of 17p, unbalanced whole-arm translocation consisting of the long arm of chromosome 17 and the short or long arm of a variable partner chromosome, unbalanced translocation resulting from a break in 17p and a break in a variable partner chromosome, dicentric chromosome resulting from a break in 17p and a break in a variable partner chromosome, monosomy 17, or balanced translocation involving chromosome 17p [t(17p;v)].
Definitions
De novo AML excludes patients with previous malignancy. AML in patients with a documented history of myelodysplasia (n = 49) or myeloproliferative neoplasm (n = 3) were considered as secondary AML (sAML). Therapy-associated myeloid neoplasm comprised patients with prior exposure to chemotherapy or radiation therapy. Upfront transplantation was defined as transplantation in first CR or during aplasia after induction therapy without previously documented refractory AML or relapse. Salvage transplantation denoted allogeneic HSCT after refractory AML had been ascertained or relapse had occurred, regardless of the remission status at transplantation. CR was defined according to the current European Leukemia Net criteria.1 The European Group for Blood and Marrow Transplantation (EBMT) score was calculated according to Gratwohl et al.17 Reduced-intensity conditioning (RIC) was defined as any regimen containing ≤10 mg/kg busulphan, ≤150 mg/m2 melphalan, or ≤8 Gray total body irradiation (TBI) and non-myeloablative conditioning (NMA) as 2 Gray TBI in combination with fludarabine. Myeloablative conditioning (MAC) were defined as >10 mg/kg busulphan, >150 mg/m2 melphalan, or >8 Gray TBI.
Statistical analysis
The goal of the collaborative approach was to collect a large number of patients with AML carrying the rare cytogenetic abnormality 17p13 in order to analyze the effect of allogeneic HSCT in subgroups large enough for reliable estimates of outcomes. Specific power considerations were not part of the study outline. Subgroup analyses, which were prespecified in the protocol, included treatment status (CR1 vs advanced disease), conditioning intensity (myeloablative conditioning vs RIC/NMA), and donor type (HLA-identical sibling vs matched unrelated and haploidentical donors).
OS, EFS, cumulative incidence of relapse (CIR), and NRM after HSCT were reported for the whole cohort, with relapse and NRM being considered as competing events. All time-dependent events were calculated from the time of transplantation. The log-rank test was used for univariate comparisons of OS and EFS. Three-year estimates are provided for all end points, together with approximative 95% CI.
Age, treatment status, MK (positive vs negative), type of AML (de novo vs other), donor type (HLA-identical sibling vs other), conditioning regimen (RIC vs MAC), and the 4 cohorts (FHCRC, HOVON, SAL, GCTSG) were selected a priori as potential confounding factors and included in multivariate Cox regression analyses. The same regression model was used to screen for center effects on the major outcomes.
Results
Patient characteristics
Data from 201 patients were analyzed. Baseline characteristics are shown in Table 1. The median age was 54 years with a range from 2 years to 75 years. Five children younger than age 18 years were included. Eighty-four patients (42%) were in CR1 at the time of HSCT. No significant differences regarding baseline characteristics were observed between those in CR1 and patients with advanced disease. Seventy patients (35%) were treated with standard MAC regimens, whereas 104 (52%) patients received RIC. Non-myeloablative conditioning was applied in 18 (9%) patients. The majority of patients (74%) received a calcineurin inhibitor in combination with methotrexate or mycophenolate mofetil for graft versus host disease (GvHD) prophylaxis.
Patient characteristics
| . | All . | CR1 . |
|---|---|---|
| . | (n = 201) (100%) . | (n = 84) (100%) . |
| Age | ||
| Median | 54 | 53 |
| Range | 2-75 | 13-75 |
| Age >60 y | 47 (23%) | 18 (22%) |
| De novo AML/sAML/t-MN | ||
| De novo | 123 (61) | 55 (65) |
| sAML | 52 (26) | 19 (23) |
| t-MN | 23 (11) | 7 (8) |
| Missing | 3 (2) | 3 (4) |
| Transplantation | ||
| Upfront | 114 (57) | 84 (100) |
| Salvage | 81 (40) | |
| Missing | 6 (3) | |
| MK | ||
| No | 43 (21) | 17 (20) |
| Yes | 155 (77) | 66 (79) |
| Missing | 3 (2) | 1 (1) |
| CK | ||
| No | 18 (9) | 6 (7) |
| Yes | 180 (90) | 77 (92) |
| Missing | 3 (2) | 1 (1) |
| Conditioning intensity | ||
| Myeloablative | 70 (35) | 31 (37) |
| Reduced intensity | 104 (52) | 35 (42) |
| Nonmyeloablative | 18 (9) | 14 (17) |
| Missing | 9 (4) | 4 (5) |
| Donor source | ||
| Sibling | 69 (34) | 29 (35) |
| Matched unrelated donor (8/8) | 86 (43) | 36 (43) |
| Partially matched unrelated donor | 36 (18) | 17 (20) |
| Haploidentical | 8 (4) | 2 (2) |
| Cord blood | 1 (1) | 0 (0) |
| Missing | 1 (1) | 0 (0) |
| Sex match | ||
| Male recipient–female donor | 32 (16) | 14 (17) |
| Any other | 109 (54) | 50 (60) |
| Missing | 60 (30) | 20 (24) |
| CMV status | ||
| Neg-neg | 44 (22) | 20 (24) |
| Pos recipient | 79 (39) | 38 (45) |
| Neg recipient–pos donor | 18 (9) | 6 (7) |
| Missing | 60 (30) | 20 (24) |
| . | All . | CR1 . |
|---|---|---|
| . | (n = 201) (100%) . | (n = 84) (100%) . |
| Age | ||
| Median | 54 | 53 |
| Range | 2-75 | 13-75 |
| Age >60 y | 47 (23%) | 18 (22%) |
| De novo AML/sAML/t-MN | ||
| De novo | 123 (61) | 55 (65) |
| sAML | 52 (26) | 19 (23) |
| t-MN | 23 (11) | 7 (8) |
| Missing | 3 (2) | 3 (4) |
| Transplantation | ||
| Upfront | 114 (57) | 84 (100) |
| Salvage | 81 (40) | |
| Missing | 6 (3) | |
| MK | ||
| No | 43 (21) | 17 (20) |
| Yes | 155 (77) | 66 (79) |
| Missing | 3 (2) | 1 (1) |
| CK | ||
| No | 18 (9) | 6 (7) |
| Yes | 180 (90) | 77 (92) |
| Missing | 3 (2) | 1 (1) |
| Conditioning intensity | ||
| Myeloablative | 70 (35) | 31 (37) |
| Reduced intensity | 104 (52) | 35 (42) |
| Nonmyeloablative | 18 (9) | 14 (17) |
| Missing | 9 (4) | 4 (5) |
| Donor source | ||
| Sibling | 69 (34) | 29 (35) |
| Matched unrelated donor (8/8) | 86 (43) | 36 (43) |
| Partially matched unrelated donor | 36 (18) | 17 (20) |
| Haploidentical | 8 (4) | 2 (2) |
| Cord blood | 1 (1) | 0 (0) |
| Missing | 1 (1) | 0 (0) |
| Sex match | ||
| Male recipient–female donor | 32 (16) | 14 (17) |
| Any other | 109 (54) | 50 (60) |
| Missing | 60 (30) | 20 (24) |
| CMV status | ||
| Neg-neg | 44 (22) | 20 (24) |
| Pos recipient | 79 (39) | 38 (45) |
| Neg recipient–pos donor | 18 (9) | 6 (7) |
| Missing | 60 (30) | 20 (24) |
CMV, cytomegalovirus; neg, negative; pos, positive; t-MN, treatment-related myeloid neoplasm.
Outcome of the whole cohort
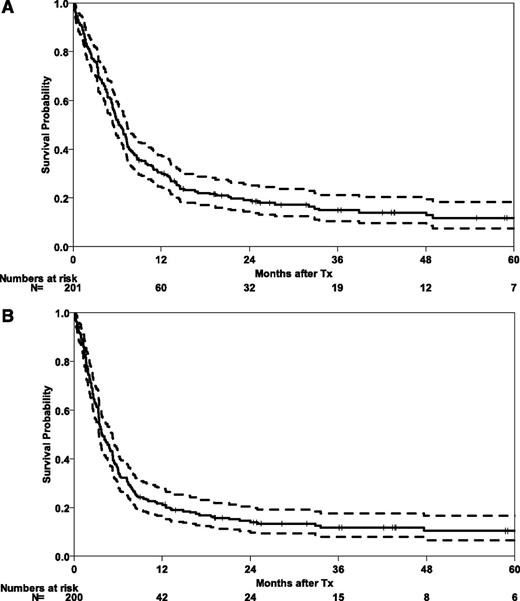
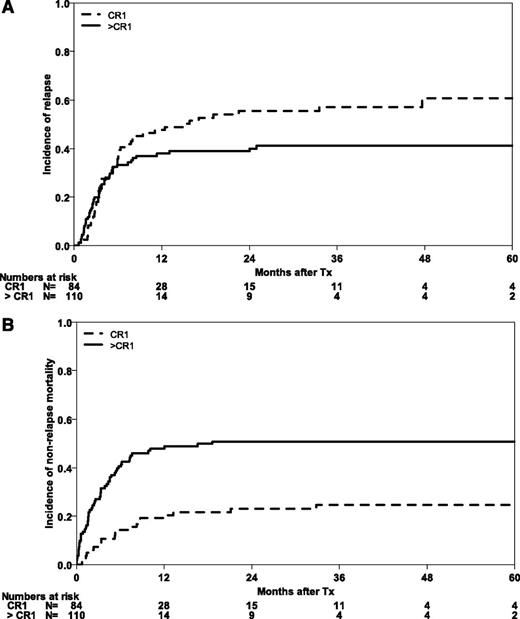
At the time of analysis, 30 patients were alive with a median follow-up of 30 months (range 1-121 months). Five-year probabilities of OS and EFS for the whole cohort are 12% (95% CI, 6-17) and 10% (95% CI, 6-15), respectively (Figure 1). Three-year outcomes are shown for the whole cohort and subgroups in Table 2. The majority of relapses occurred during the first months after HSCT, with a CIR for the whole cohort of 37% (95% CI, 30-43) at 6 months. After 5 years, CIR reached 50% (95% CI, 43-58). Early NRM was substantial at 29% (95% CI, 23-35), mainly in those patients with advanced disease (NRM of 40% [95% CI, 31-49], as compared with 14% [95% CI, 7-22] for patients in CR1; Figure 2).
Survival outcomes after HSCT in abnl(17p) AML. OS (A) and EFS (B) after HSCT in 201 patients with abnl(17p) AML. Tx, treatment.
Survival outcomes after HSCT in abnl(17p) AML. OS (A) and EFS (B) after HSCT in 201 patients with abnl(17p) AML. Tx, treatment.
Outcome at 3 year post-HSCT by risk factors
| . | Frequency . | OS . | EFS . | CIR . | NRM . |
|---|---|---|---|---|---|
| . | n . | OS % (95%CI) . | EFS % (95%CI) . | CIR % (95%CI) . | NRM % (95%CI) . |
| All patients | 201 | 15 (10-20) | 12 (7-16) | 49 (42-56) | 39 (32-46) |
| Age | |||||
| <40 | 29 | 20 (5-35) | 14 (1-26) | 52 (33-71) | 34 (17-52) |
| 40-50 | 44 | 23 (10-37) | 19 (6-32) | 51 (36-66) | 30 (15-48) |
| 50-60 | 80 | 10 (4-17) | 8 (2-14) | 47 (36-66) | 45 (34-56) |
| >60 | 48 | 10 (0-21) | 11 (1-21) | 48 (32-64) | 40 (26-54) |
| P* | .029 | .1 | .9 | .2 | |
| Date of HSCT | |||||
| Before median† | 100 | 9 (3-15) | 7 (2-12) | 54 (44-64) | 39 (30-49) |
| After median | 101 | 22 (13-31) | 18 (10-26) | 43 (33-53) | 38 (29-48) |
| P* | .048 | .2 | .2 | .9 | |
| CR | |||||
| CR1 | 84 | 22 (13-32) | 18 (10-27) | 57 (46-68) | 25 (15-34) |
| >CR1 | 111 | 9 (3-15) | 7 (2-13) | 41 (32-51) | 51 (41-60) |
| P* | <.001 | <.001 | .08 | <.001 | |
| Missing | 6 | ||||
| Type of abnl(17p) | |||||
| add(17p) | 30 | 20 (4-36) | 20 (6-34) | 53 (35-72) | 27 (10-43) |
| i(17q) | 11 | 18 (0-41) | 9 (0-26) | 55 (22-88) | 36 (3-69) |
| del(17p) | 16 | NA | NA | NA | NA |
| der(17v)(q10;v)/der(17)t(17p;v) | 29 | 19 (2-35) | 14 (0-27) | 31 (12-51) | 53 (34-72) |
| dic(17p;v) | 12 | 25 (1-49) | 17 (0-38) | 42 (11-73) | 42 (11-72) |
| −17 | 86 | 10 (3-17) | 11 (4-18) | 54 (43-65) | 35 (25-46) |
| Balanced t(17p;v) | 4 | NA | NA | NA | NA |
| P* | .2 | .5 | .4 | .2 | |
| Missing | 13 | ||||
| Sub classification of CK | |||||
| <3 abnl. | 18 | 28 (7-48) | 22 (3-41) | 33 (10-56) | 44 (20-69) |
| 3-4 abnl. | 24 | 30 (11-50) | 22 (4-40) | 55 (34-77) | 23 (4-41) |
| >4 abnl. | 151 | 11 (5-16) | 9 (4-13) | 50 (42-58) | 41 (33-49) |
| P* | .01 | .02 | .2 | .1 | |
| Missing§ | 8 | ||||
| MK | |||||
| MK− | 43 | 29 (15-44) | 20 (7-33) | 46 (30-62) | 34 (19-49) |
| MK+ | 155 | 11 (5-16) | 9 (4-14) | 50 (42-58) | 41 (33-48) |
| P* | .003 | .004 | .3 | .3 | |
| Missing | 3 | ||||
| Conditioning regimen | |||||
| RIC | 104 | 16 (8-24) | 13 (6-21) | 44 (34-53) | 42 (32-53) |
| MAC | 70 | 12 (4-20) | 8 (2-15) | 53 (41-65) | 39 (27-51) |
| NMA | 18 | 27 (6-48) | 22 (3-41) | 61 (37-85) | 17 (0-35) |
| P* | .5 | .9 | .07 | .1 | |
| Missing | 9 | ||||
| TBI | |||||
| No TBI | 99 | 13 (5-20) | 12 (5-20) | 47 (37-57) | 40 (29-50) |
| ≤8 Gy TBI | 51 | 21 (9-32) | 18 (7-28) | 53 (39-67) | 29 (17-42) |
| >8 Gy TBI | 42 | 17 (5-28) | 7 (0-15) | 47 (31-62) | 46 (30-62) |
| P* | .3 | .8 | .2 | .2 | |
| Missing | 9 | ||||
| Donor type | |||||
| Sibling | 69 | 17 (8-27) | 16 (7-25) | 49 (37-62) | 34 (22-45) |
| MUD | 86 | 19 (10-27) | 10 (2-18) | 54 (42-66) | 36 (26-47) |
| MMUD | 36 | 10 (0-20) | 10 (0-21) | 44 (28-61) | 45 (28-62) |
| Haplo | 8 | NA | NA | NA | NA |
| Cord blood | 1 | NA | NA | NA | NA |
| P* | .6 | .7 | .6 | .1 | |
| Missing | 1 |
| . | Frequency . | OS . | EFS . | CIR . | NRM . |
|---|---|---|---|---|---|
| . | n . | OS % (95%CI) . | EFS % (95%CI) . | CIR % (95%CI) . | NRM % (95%CI) . |
| All patients | 201 | 15 (10-20) | 12 (7-16) | 49 (42-56) | 39 (32-46) |
| Age | |||||
| <40 | 29 | 20 (5-35) | 14 (1-26) | 52 (33-71) | 34 (17-52) |
| 40-50 | 44 | 23 (10-37) | 19 (6-32) | 51 (36-66) | 30 (15-48) |
| 50-60 | 80 | 10 (4-17) | 8 (2-14) | 47 (36-66) | 45 (34-56) |
| >60 | 48 | 10 (0-21) | 11 (1-21) | 48 (32-64) | 40 (26-54) |
| P* | .029 | .1 | .9 | .2 | |
| Date of HSCT | |||||
| Before median† | 100 | 9 (3-15) | 7 (2-12) | 54 (44-64) | 39 (30-49) |
| After median | 101 | 22 (13-31) | 18 (10-26) | 43 (33-53) | 38 (29-48) |
| P* | .048 | .2 | .2 | .9 | |
| CR | |||||
| CR1 | 84 | 22 (13-32) | 18 (10-27) | 57 (46-68) | 25 (15-34) |
| >CR1 | 111 | 9 (3-15) | 7 (2-13) | 41 (32-51) | 51 (41-60) |
| P* | <.001 | <.001 | .08 | <.001 | |
| Missing | 6 | ||||
| Type of abnl(17p) | |||||
| add(17p) | 30 | 20 (4-36) | 20 (6-34) | 53 (35-72) | 27 (10-43) |
| i(17q) | 11 | 18 (0-41) | 9 (0-26) | 55 (22-88) | 36 (3-69) |
| del(17p) | 16 | NA | NA | NA | NA |
| der(17v)(q10;v)/der(17)t(17p;v) | 29 | 19 (2-35) | 14 (0-27) | 31 (12-51) | 53 (34-72) |
| dic(17p;v) | 12 | 25 (1-49) | 17 (0-38) | 42 (11-73) | 42 (11-72) |
| −17 | 86 | 10 (3-17) | 11 (4-18) | 54 (43-65) | 35 (25-46) |
| Balanced t(17p;v) | 4 | NA | NA | NA | NA |
| P* | .2 | .5 | .4 | .2 | |
| Missing | 13 | ||||
| Sub classification of CK | |||||
| <3 abnl. | 18 | 28 (7-48) | 22 (3-41) | 33 (10-56) | 44 (20-69) |
| 3-4 abnl. | 24 | 30 (11-50) | 22 (4-40) | 55 (34-77) | 23 (4-41) |
| >4 abnl. | 151 | 11 (5-16) | 9 (4-13) | 50 (42-58) | 41 (33-49) |
| P* | .01 | .02 | .2 | .1 | |
| Missing§ | 8 | ||||
| MK | |||||
| MK− | 43 | 29 (15-44) | 20 (7-33) | 46 (30-62) | 34 (19-49) |
| MK+ | 155 | 11 (5-16) | 9 (4-14) | 50 (42-58) | 41 (33-48) |
| P* | .003 | .004 | .3 | .3 | |
| Missing | 3 | ||||
| Conditioning regimen | |||||
| RIC | 104 | 16 (8-24) | 13 (6-21) | 44 (34-53) | 42 (32-53) |
| MAC | 70 | 12 (4-20) | 8 (2-15) | 53 (41-65) | 39 (27-51) |
| NMA | 18 | 27 (6-48) | 22 (3-41) | 61 (37-85) | 17 (0-35) |
| P* | .5 | .9 | .07 | .1 | |
| Missing | 9 | ||||
| TBI | |||||
| No TBI | 99 | 13 (5-20) | 12 (5-20) | 47 (37-57) | 40 (29-50) |
| ≤8 Gy TBI | 51 | 21 (9-32) | 18 (7-28) | 53 (39-67) | 29 (17-42) |
| >8 Gy TBI | 42 | 17 (5-28) | 7 (0-15) | 47 (31-62) | 46 (30-62) |
| P* | .3 | .8 | .2 | .2 | |
| Missing | 9 | ||||
| Donor type | |||||
| Sibling | 69 | 17 (8-27) | 16 (7-25) | 49 (37-62) | 34 (22-45) |
| MUD | 86 | 19 (10-27) | 10 (2-18) | 54 (42-66) | 36 (26-47) |
| MMUD | 36 | 10 (0-20) | 10 (0-21) | 44 (28-61) | 45 (28-62) |
| Haplo | 8 | NA | NA | NA | NA |
| Cord blood | 1 | NA | NA | NA | NA |
| P* | .6 | .7 | .6 | .1 | |
| Missing | 1 |
del(17p), deletion of 17p; der(17)t(17p;v), unbalanced translocation resulting from a break in 17p and a break in a variable partner chromosome; dic(17p;v), dicentric chromosome resulting from a break in 17p and a break in a variable partner chromosome; Gy, Gray; i(17q), isochromosome for the entire long arm of one chromosome 17; MMUD, partially matched unrelated donor; MUD, matched unrelated donor; NA, not available.
P values were calculated using the Gray test for CIR and NRM and the log-rank test for OS and EFS.
The median date of performed transplantations was October 26, 2006.
Deletion of TP53 by FISH only.
Because of the combination of conventional karyotyping and FISH analysis, no exact number of aberrations could be denoted in 8 patients.
Causes of treatment failure after HSCT in abnl(17p) AML. CIR (A) and NRM (B) according to remission status (CR1 vs advanced stages).
Causes of treatment failure after HSCT in abnl(17p) AML. CIR (A) and NRM (B) according to remission status (CR1 vs advanced stages).
The incidence of acute GvHD grades II to IV up to day 100 was 32% (95% CI, 25-38) and 11% (95% CI, 6-15) for grades III to IV. With death and relapse as competing events, the cumulative incidence for chronic GvHD up to 1 year was remarkably low with 8% (95% CI, 4-12).
Type of abnl(17p)
Abnormalities of 17p13 in AML comprise a heterogeneous group of structural and numerical chromosome abnormalities. Monosomy 17, der(17p), and add(17p) were the most prevalent abnormalities in this specific cohort of patients. Because monosomies are associated with the loss or shattering of the entire chromosome, we considered the possibility that “lost” chromosomal 17 material could have been integrated into other chromosomes and remained functional, such as in derivative or marker chromosomes. Conventional banding techniques cannot exclude this possibility18 ; therefore, we sought for differences in the outcome of the various types of cytogenetic lesions. Notably, no significant differences were observed in patients with different types of abnormalities of 17p13 with respect to survival and relapse incidence (Table 2). Balanced translocations as a sole abnormality were reported for only 4 patients (2%), whereas the AML genomes of 173 patients (86%) contained 3 or more abnormalities.
Complex and MK
Only 18 patients (9%) did not meet the criteria of a CK. Three of these had an additional high-risk abnormality, 1 each with −7, del(5q), and t(6;11)(q27;q23). In those patients in whom abnl(17p) constituted the only cytogenetic high-risk abnormality, EFS at 3 years was 27% (95% CI, 4-49). No difference with respect to EFS and OS was noted between those 18 patients who did not have CK AML and 24 patients with CK harboring 3 or 4 cytogenetic abnormalities. Patients with more than 4 abnormalities had significantly worse survival compared with the remaining patients (P = .007).
Classification according to MK is an alternative way to identify ultra-high-risk cytogenetic abnormalities. With an OS at 3 years of 29% (95% CI, 15-44) the 43 patients (21%) without MK had a significantly (P = .003) better outcome compared with those who possessed an MK with an OS of 11% (95% CI, 5-16). However, relapse incidence and nonrelapse mortality were not independently statistically significant when comparing MK and CK positive AMLs.
Transplant outcome in CR1
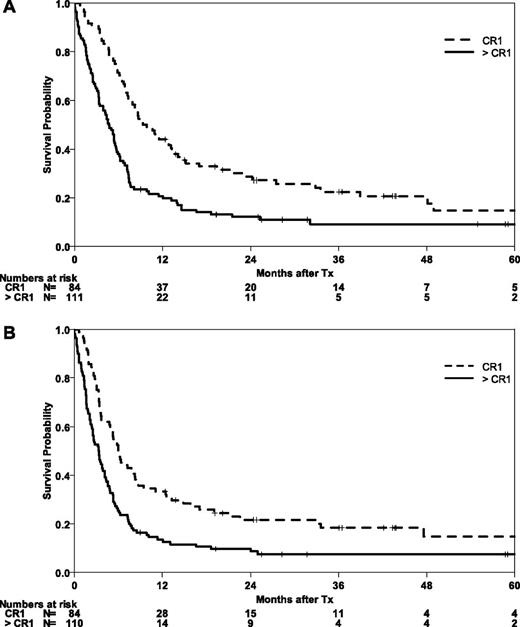
Patients who were in CR1 at the time of transplantation had higher OS and EFS than those with advanced disease (P = .001) (Figure 3). At 5 years, the probabilities of OS and EFS were 15% (95% CI, 5-24) for both survival end points. Relapse was an early event even in this group of patients, with a CIR of 38% (95% CI, 28-49) at 6 months. The incidence of relapse increased to 61% (95% CI, 48-73) at 5 years in this group of CR-1 patients. Altogether, 67% of relapses in this group were observed in the first 6 months. Expectedly, NRM was lower in this group of patients who had received less chemotherapy compared with the group of patients with advanced disease (P = .001). The incidence of NRM at 6 months was 14% (95% CI, 7-22) and reached 25% (95% CI, 15-34) at 5 years.
Impact of remission status prior to HSCT in abnl(17p) AML. OS (A) and EFS (B) after HSCT according to remission status (CR1 vs advanced stages).
Impact of remission status prior to HSCT in abnl(17p) AML. OS (A) and EFS (B) after HSCT according to remission status (CR1 vs advanced stages).
Impact of additional risk factors and scores
As shown in Table 2, neither donor type nor the intensity of the conditioning regimen, or the use of TBI had a significant influence on OS, EFS, or CIR. Interestingly, this also applied to NRM. All 8 patients who had received transplants from haploidentical donors died, 2 subsequent to relapse and 6 from NRM. In a multivariate model including age, treatment status, type of AML, donor type, and conditioning regimen only age (P = .01), treatment status (P = .003) and MK (P = .04) had a significant impact on OS. Center effects were not revealed.
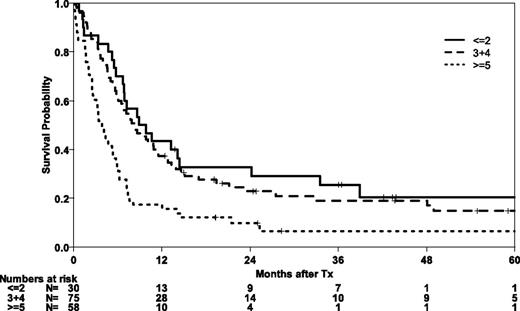
Post hoc, we attempted to analyze the performance of a comprehensive risk score for transplantation in a subset of 163 patients with available information on all risk factors. The EBMT risk score integrates information on age, disease stage, time interval from diagnosis to transplant, donor type, and donor-recipient sex combination. Patients with lower risk scores had significantly better OS and EFS (P = .001 for both end points) (Figure 4). The 5-year probability of OS in the best risk group was 20% (95% CI, 5-36).
Finally, we investigated the baseline characteristics of 24 patients who were disease-free at 2 years after transplantation. Twenty-one of them (88%) received allogeneic HSCT as first-line consolidating therapy. No significant differences regarding age, type of AML, conditioning intensity, or donor type were observed between long-term survivors and failures. Clustering of a specific 17p abnormality in this subset was ruled out. However, MK (χ-square test, P = .002) and CK (χ-square test, P = .004) were less frequent among these patients with better long-term disease control. Detailed information on the baseline characteristics is provided in supplemental Table 1 on the Blood Web site.
Discussion
High-risk karyotypes in AML comprise a variety of different chromosome abnormalities characterized by distinct lesions or by summary measures of genomic instability, such as CK or MK. To what extent patients with distinct high-risk lesions benefit from allogeneic HSCT is unknown. Here, we present data on 201 patients who underwent allogeneic HSCT during the past decade and harbored abnl(17p) in their AML karyotype. This subgroup is of special interest because cytogenetic abnormalities affecting the integrity of 17p13 presumably indicate the worst genetic subgroup in AML as suggested by several retrospective reports.9,16,19,20 Thus, one important question is whether sufficient evidence exists that patients with this specific abnormality benefit from allogeneic HSCT.
OS and EFS after allogeneic HSCT in CR1 was 15% after 5 years (95% CI, 5-24). When interpreting this result, it has to be taken into account that 23% of patients were transplanted at an age older than 60 years and 22% of patients were transplanted from partially matched unrelated donors. Information on the transplant-specific risk profile, as reflected in the EBMT risk score, indicates that low transplant-risk patients with abnl(17p) AML had significantly better OS and EFS (P = .001).17 Patients in the best risk category had a 5-year OS of 20% (95% CI, 5-36). Together with information on better outcome of patients whose AML karyotype did not meet the criteria of a MK and who were transplanted in recent years, or in CR1, this observation argues against an intrinsic resistance of abnl(17p) AML to allogeneic graft-versus-leukemia effects. Still, the overall outcome after allogeneic HSCT is disappointing.
Because subgroup analyses of rare genetic high-risk abnormalities were not part of the prospective studies that established the role of allogeneic HSCT in high-risk AML, retrospective analyses using pooled registry data, here derived from 2 local transplant registries and randomized controlled trials, are reasonable approaches to study the effect of transplantation in the respective genetic subgroups.
The only direct evaluation of the value of allogeneic HSCT in abnl(17p) AML is a retrospective as-treated analysis with pooled data from 3 randomized SAL trials. All trials encompassed a risk-adapted transplant strategy for patients with high-risk karyotype AML. A beneficial effect of allogeneic HSCT could not be demonstrated in this analysis. One of 21 patients who received postremission therapy achieved long-term disease control, translating into 3-year leukemia-free survival of 5% (95% CI, 0-14). Strikingly, all 16 patients who proceeded to allogeneic HSCT in CR1 died within 39 months, mainly after relapse.14 Further information comes from reports on TP53 mutated AML. Bowen et al published data on TP53-mutated AML in 61 patients who were enrolled into Medical Research Council AML clinical trials. Their median age was 64 years and the majority of patients received intensive chemotherapy. All patients with mutant TP53 died within 2 years from enrollment.20 Grossmann et al reported a median 4.6 months of survival from first diagnosis in 80 patients with TP53-mutated AML and 3-year EFS and OS estimates of 0%.16 When comparing the results after allogeneic HSCT with published data on the outcome of abnl(17p)/TP53-mutated AML, that a small fraction of good-risk patients achieves long-term disease control after allogeneic HSCT may be interpreted as an indication of the efficacy of allogeneic HSCT. However, taking the limitations of historical comparisons into consideration, this statement provides only modest evidence for the efficacy of allogeneic HSCT.
The incidence of cGvHD up to 1 year after HSCT was only 8% in this cohort of patients, likely because we considered relapse before the first occurrence of chronic GVHD as a competing event. The justification of this approach is that chronic GVHD is regarded here as surrogate for a potential graft-versus-leukemia effect. The low rate of chronic GVHD can thus be explained by the high incidence of relapse as a competing event, especially because almost 70% of all relapses occurred within the first 6 months. The low incidence of chronic GVHD thus indicates that the desired immunologic mechanism of HSCT may not have come into effect. This observation could argue for a more aggressive taper of immunosuppressive drugs or administration of prophylactic donor lymphocyte infusion.21
Although this approach is limited by the risk of GvHD, prophylactic or preemptive pharmacologic approaches that use immune modulation by hypomethylating agents might be more attractive.22 Platzbecker et al reported on responses in patients with TP53-mutated MDS after treatment with lenalidomide and azacytidine, demonstrating the feasibility and efficacy of preemptive treatment with azacytidine after allogeneic HSCT, as triggered by minimal residual disease markers even in patients with GvHD or ongoing immunosuppressive therapy.23,24 Similarly, Bug et al recently reported promising preliminary findings with the prophylactic administration of the orally available pan-histone deacetylase inhibitor panobinostat after allogeneic HSCT in patients with high-risk AML or MDS.25
No significant difference in outcome was noted in multivariate analysis for the type of conditioning. This finding is in line with a recently published randomized study comparing a reduced vs standard intensity TBI-based conditioning in patients with AML, in which no differential effects of the dose-intensity were also noted with respect to the cytogenetic risk.26 Further, in line with p53-dependence of irradiation induced cell death, the clinical data do not suggest that the use of TBI has a favorable impact. Still, more efficient preparative regimens based on drugs that allow for p53-independent cell killing would be desirable. Similarly, the finding that donor type is not associated with any survival end point in this subset is compatible with the published literature.27,28
In conclusion, patients with abnl(17p) AML have a poor outcome even after allogeneic HSCT. Nevertheless, in CR1, allogeneic HSCT remains the treatment of choice for good-risk transplant candidates lacking promising alternatives. Generally, more efficient treatment strategies, which, for example, could aim at inducing p53-independent cell death, are urgently required for this subgroup of patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gary Schoch, Fred Hutchinson Cancer Research Center, for his assistance in querying the transplant database; Yvette van Norden, Department of Trials and Statistics, Erasmus MC Rotterdam, for her assistance in exporting data from databases; and Christian Klesse, German Bone Marrow Donor Center, Clinical Trials Unit, Dresden, Germany, for supporting the data management process.
Authorship
Contribution: G.E. and M.B. provided financial and administrative support; J.M.M. and J. Schetelig designed the study; J.M.M., M.F., J.J.C., F.R.A., M.S., H.B., G.B., K.S.-E., U.H., T.B., R.B.W., C.R., B.M., B.L., and J. Sanz collected the cytogenetic and clinical data; J.M.M., B.M., F.S., J.J.C., M.F., F.R.A., R.B.W., B.L., M.B., and J. Schetelig analyzed and interpreted the data; and all authors contributed to the writing process and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes Schetelig, Medical Department I, University Hospital Carl Gustav Carus, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: johannes.schetelig@uniklinikum-dresden.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal