Key Points
Z-BEAM/C did not improve outcome for patients in only PR or CRu before transplant.
Positive PET before transplant and MRD after transplant predicted inferior PFS and OS.
Abstract
The main objective of the MCL3 study was to improve outcome for patients not in complete remission (CR) before transplant by adding 90Y-ibritumomab-tiuxetan (Zevalin) to the high-dose regimen. One hundred sixty untreated, stage II-IV mantle cell lymphoma patients <66 years received rituximab (R)–maxi–CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone) alternating with R-high-dose cytarabine (6 cycles total), followed by high-dose BEAM/C (bis-chloroethylnitrosourea, etoposide, cytarabine, and melphalan or cyclophosphamide) and autologous stem cell transplantation from 2005 to 2009. Zevalin (0.4 mCi/kg) was given to responders not in CR before transplant. Overall response rate pretransplant was 97%. The outcome did not differ from that of the historic control: the MCL2 trial with similar treatment except for Zevalin. Overall survival (OS), event-free survival (EFS), and progression-free survival (PFS) at 4 years were 78%, 62%, and 71%, respectively. For responding non-CR patients who received Zevalin, duration of response was shorter than for the CR group. Inferior PFS, EFS, and OS were predicted by positron emission tomography (PET) positivity pretransplant and detectable minimal residual disease (MRD) after transplant. In conclusion, positive PET and MRD were strong predictors of outcome. Intensification with Zevalin may be too late to improve the outcome of patients not in CR before transplant. This trial was registered at www.clinicaltrials.gov as #NCT00514475.
Introduction
Mantle cell lymphoma (MCL) comprises 5% to 10% of non-Hodgkin lymphomas (NHL) and often has an aggressive clinical course. At diagnosis, the disease is typically in stage III-IV with universal lymphadenopathy and bone marrow involvement.1 Other extranodal sites like the gastrointestinal tract are often involved.2 In addition to a characteristic immunophenotype, the hallmark chromosomal translocation t(11:14) leading to aberrant expression of cyclin D1 can be found in most cases.3 The importance of an effective induction regimen containing both cytarabine and rituximab has gained support based on results from phase 2 and 3 trials.4-7 MCL is highly radiosensitive and anti-CD20–targeted radioimmunotherapy with 90Y-ibritumomab-tiuxetan (Zevalin) or 131I-tositumomab (Bexxar) has been shown to be effective and well tolerated.8-10 Because myelosuppression is the major toxicity of these strategies, standard-dose radioimmunotherapy plus myeloablative chemotherapy followed by stem cell rescue has been tested with promising results in MCL and other NHL.8,11
The Nordic phase 2 MCL2 study, which included 160 patients, showed a strong correlation between the response pretransplant and long-term progression-free survival (PFS) and overall survival (OS). Hence, in the present study (MCL3), responders who did not achieve a complete remission (CR) but only unconfirmed CR (CRu) or partial remission (PR) after induction immunochemotherapy were offered late intensification with standard-dose Zevalin (0.4 mCi/kg) prior to high-dose treatment with BEAM (bis-chloroethylnitrosourea, etoposide, cytarabine, and melphalan) or BEAC (bis-chloroethylnitrosourea, etoposide, cytarabine, and cyclophosphamide) (Z-BEAM/C). The main objective was to improve duration of response in this patient subset. We further aimed to evaluate the prognostic value of positron emission tomography (PET) and minimal residual disease (MRD) analysis.
Patients and methods
Patients, diagnostic work-up, and follow-up
The Nordic Lymphoma Group has conducted 3 successive phase 2 trials since 1996, including the present MCL3 study which recruited 160 patients from 2005 to 2009. The eligibility criteria were identical: previously untreated stage II-IV MCL patients <66 years. The diagnostic specimens should fulfill the World Health Organization (WHO) criteria for MCL3 regarding immunophenotype, cyclin D1 overexpression, or t(11;14) by central pathology review. Patients underwent standard clinical and laboratory work-up including computed tomography (CT) scans of chest and abdomen, bone marrow biopsy, and aspirates for histology and flow cytometry. Blood and bone marrow aspirates were sent to a central laboratory (Leukemia Marker Laboratory, Department of Hematology, Rigshospitalet, Copenhagen, Denmark) for identification of disease- or patient-specific molecular markers. MCL International Index (MIPI) and MIPI-biologic (MIPI-B) scores12 were assessed at inclusion. The complete work-up was repeated after the fifth cycle of induction therapy and 3 months after the transplant. A PET/CT scan was done after cycle 5 whenever feasible, especially for patients who had not achieved CR by regular CT scan, but should not influence treatment choice or response evaluation. PET scans were centrally reviewed in Sweden, Finland, and Norway and in Denmark by experts at 4 large university clinics. The scans were scored according to the Deauville13 criteria with a 5-point scale, where scores of 4 or 5 were considered positive. PET/CT scan was not performed in the MCL2 study. Follow-up visits were done every 6 months for 5 years with clinical examination, CT scans, and blood and bone marrow samples. After 5 years, patients in remission continued annual follow-up with clinical assessment and blood samples. The Nordic MCL3 protocol was approved by all relevant medicine agencies and ethics committees. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Treatment
Induction therapy for the MCL2 and MCL3 trials consisted of a total of 6 cycles of alternating maxi–CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone)–rituximab and high-dose cytarabin–rituximab administered every third week with granulocyte colony-stimulating factor support.5 Patients responding after 5 cycles underwent stem cell mobilization and peripheral blood stem cell harvest after the sixth cycle (cytarabin–rituximab). For in vivo purging, an extra dose of rituximab was administered at day 9 in cycle 6. A minimum of 2 × 106 CD34+ cells per kilogram was required to proceed to high-dose therapy with stem cell support. Responders who only achieved CRu or PR after cycle 5 were offered late intensification with a standard dose of Zevalin (90Y-ibritumomab-tiuxetan, 0.4 mCi/kg; maximum, 32 mCi) 1 week before the start of high-dose chemotherapy. Rituximab 250 mg/m2 was given both 1 week before and just prior to Zevalin. As described for MCL2,5 BEAM or BEAC conditioning was then given, followed by stem cell infusion. During follow-up, patients who were MRD positive after transplant and showed an increase in polymerase chain reaction (PCR) signal and PCR-negative patients who converted to PCR positivity without signs of clinical relapse were offered preemptive treatment with rituximab 375 mg/m2 weekly for 4 weeks.
Detection of MRD
As described elsewhere,5 fresh samples of blood and bone marrow were shipped overnight from all centers to the central laboratory in Copenhagen. Briefly, DNA was extracted and used for PCR primer design and standard nested PCR amplification of patient-specific clonally rearranged immunoglobulin heavy chain (IGHV) genes and/or Bcl-1/IGHV rearrangement (translocation 11;14). Blood and bone marrow analyses were then performed after the fifth cycle of induction treatment, 3 and 6 months after transplant and then every 6 months to detect MRD and molecular relapse. PCR-positive follow-up samples were sequenced to secure identity with the original IGHV sequence/t(11;14).
Response criteria, end points, and statistics
Response, event-free survival (EFS), PFS, OS, and response duration were assessed according to National Cancer Institute (NCI) criteria.14 Response duration was calculated for responding patients who completed the therapy, from the date of first documentation of response until the date of relapse or progression of lymphoma. Survival analyses were performed according to the Kaplan-Meier method15 and differences between subgroups were analyzed by the log-rank test. Multivariate Cox regression analysis was performed to assess the effect of prognostic factors on outcome.
Results
Between 2005 and 2009, 162 previously untreated patients, 18 to 65 years of age, were recruited. The inclusion and exclusion criteria were the same as for the previously reported MCL2 trial.5 At central pathology review only 1 case was excluded as non-MCL lymphoma. Another patient was excluded due to a concomitant renal cell carcinoma, leaving 160 evaluable patients. The patient characteristics were typical for MCL with a median age of 58 years, the majority in stage IV with bone marrow infiltration, and a typical distribution between MIPI risk groups and a pattern of Ki67 expression (Table 1).
Characteristics of patients in MCL3 compared with MCL2
| Variable . | MCL3 . | MCL2 . | P . |
|---|---|---|---|
| Male sex (%) | 129 (80) | 113 (71) | .05 |
| Median age (range), y | 58 (28-65) | 56 (32-65) | |
| Stage IV (%) | 139 (88) | 136 (85) | .37 |
| MIPI score (%) | |||
| Low | 77 (48) | 80 (51) | .75 |
| Intermediate | 49 (31) | 41 (26) | |
| High | 33 (21) | 37 (23) | |
| Cytologic variant (%) | |||
| Blastoid | 28 (18) | 31 (19) | .36 |
| Common | 128 (82) | 129 (81) | |
| % Ki67 (%) | |||
| 0-9 | 11 (9) | 10 (8) | .94 |
| 10-29 | 61 (49) | 60 (50) | |
| >29 | 53 (42) | 50 (42) |
| Variable . | MCL3 . | MCL2 . | P . |
|---|---|---|---|
| Male sex (%) | 129 (80) | 113 (71) | .05 |
| Median age (range), y | 58 (28-65) | 56 (32-65) | |
| Stage IV (%) | 139 (88) | 136 (85) | .37 |
| MIPI score (%) | |||
| Low | 77 (48) | 80 (51) | .75 |
| Intermediate | 49 (31) | 41 (26) | |
| High | 33 (21) | 37 (23) | |
| Cytologic variant (%) | |||
| Blastoid | 28 (18) | 31 (19) | .36 |
| Common | 128 (82) | 129 (81) | |
| % Ki67 (%) | |||
| 0-9 | 11 (9) | 10 (8) | .94 |
| 10-29 | 61 (49) | 60 (50) | |
| >29 | 53 (42) | 50 (42) |
Study-terminating events, EFS, and OS
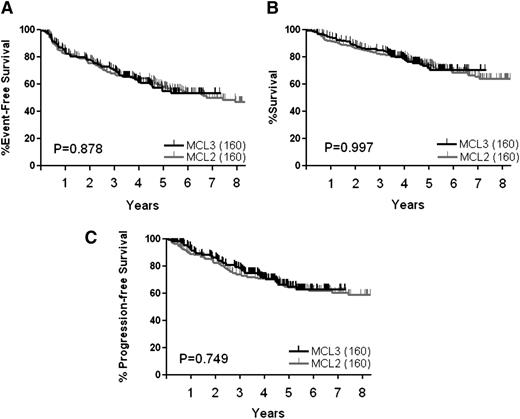
With a median observation time of 4.4 years, study-terminating events have occurred in 67 patients (42%): in 48 (30%) due to lack of response, relapse, or progression; in 6 (4%) due to harvest failure; in 3 (2%) due to toxicity; and in 10 (6%) due to death from other causes than MCL during treatment or after. The 4-year EFS for the MCL3 cohort (n = 160) was 62% (Figure 1A). Forty-one patients have died, 30 of lymphoma and 11 of other causes: 1 sudden death after induction cycle 1, 3 from transplant complications (BEAM/C, 2; Z-BEAM/C, 1), 3 from secondary myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML), and 4 from other causes during follow-up (1 each of: suicide, accident, large bowel cancer, infection). The 4-year OS rate for the patients in the MCL3 study (n = 160) was 78% (Figure 1B).
Survival curves for MCL2 and MCL3 based on intention to treat of all patients. (A) EFS, (B) OS, (C) PFS.
Survival curves for MCL2 and MCL3 based on intention to treat of all patients. (A) EFS, (B) OS, (C) PFS.
Response and PFS
At the first response evaluation after 5 cycles of induction therapy, prior to transplant, 155 of 160 patients (97%) had responded to the induction treatment: 82 (51%) had achieved CR, 26 (16%) CRu, and 47 (29%) PR. Of the 155 responders, 146 proceeded to transplant, whereas 9 did not (6 harvest failure, 3 toxicity). After the transplant, the number of patients in CR had increased to 119 (82%), 13 (9%) remained in CRu, 6 (4%) in PR, 4 (3%) had progressed, and 4 (3%) had died. A total of 52 patients have relapsed or progressed during induction or follow-up and this translates into a 4-year PFS of 71% (Figure 1C). No relapses have so far occurred among the 34 patients observed in remission beyond 5 years after transplant.
PET scan prior to transplant predicts outcome
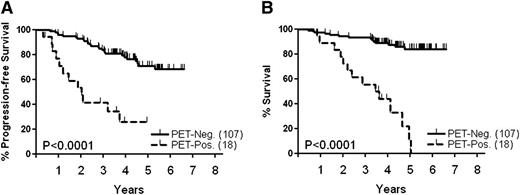
PET/CT scanning was performed in 125 patients after cycle 5 of the induction treatment and 18 patients (14%) were scored PET positive. As expected, patients who were in PR by regular CT scans were more often PET positive compared with patients in CRu (36% vs 8%). Only 1 patient in CR according to the NCI criteria of 199914 had a positive PET scan. Thirteen of 18 PET-positive patients (72%) have progressed/relapsed, compared with 26 of 107 (23%) PET-negative patients (P = .017). With a median observation time of 4.4 years, the 4-year PFS of the PET-positive cohort was 27%, significantly worse than the 78% of the PET-negative group (Figure 2A, P < .0001). This translated into inferior OS for patients who had a positive PET scan before transplant (Figure 2B, P < .0001).
Survival of patients according to results of PET-scan prior to transplant. (A) PFS, (B) OS.
Survival of patients according to results of PET-scan prior to transplant. (A) PFS, (B) OS.
Detection of MRD before and after transplant
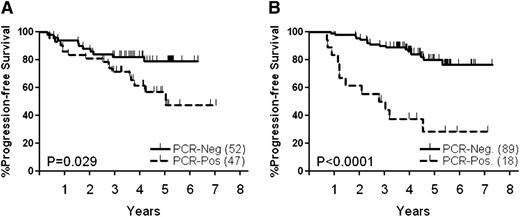
Prior to the transplant, 47 of 99 patients (47%) were MRD positive in blood and/or bone marrow, compared with only 18 of 107 (17%) patients tested after transplant. Of patients tested at both time points, 26 of the MRD-positive cases had converted to MRD negative posttransplant, suggesting that high-dose therapy improved the quality of the remission. Patients who were MRD positive prior to transplant (Figure 3A, P = .029) or after transplant (Figure 3B, P = .0001) had significantly shorter PFS than patients who were MRD negative. Among the 18 patients who were MRD positive posttransplant, 12 (67%) have relapsed, compared with only 16 of 89 patients (18%) in the MRD-negative group (P < .01). Of note, 10 of the 14 PET-positive patients who had a PCR primer were also MRD positive before transplant, 8 of whom have relapsed.
PFS according to results of MRD analysis. (A) Before transplant, (B) after transplant.
PFS according to results of MRD analysis. (A) Before transplant, (B) after transplant.
Prognostic factors
Similar to the MCL2 study population, MIPI and MIPI-B were strong predictors for outcome.16 The 5-year survival of MIPI low- and intermediate-risk groups did not differ significantly (82% and 72%, respectively) although it was 50% for the MIPI high-risk patients (not shown). Of MIPI-B variables (age, WHO, white blood cell count [WBC], lactate dehydrogenase [LDH], Ki67) only WHO performance status was shown to be an independent prognostic factor for EFS and LDH for OS in multivariate analyses (Table 2). In addition, a blastoid type of MCL was significant for inferior PFS and OS. In patients with available MRD primer and PET scan performed, MIPI-B, a positive PET pretransplant, and detectable MRD posttransplant were independent variables for EFS, PFS, and OS (Table 3). When performing a multivariate analysis based on pooled data from the MCL2 and MCL3 studies, treatment with Zevalin did not come out as an independent prognostic factor for EFS, PFS, or OS (not shown).
Multivariate analysis of 142 patients with available Ki67 value according to outcome
| Variable . | Score . | EFS . | PFS . | Survival . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Age | Value | 0.99 | 0.96-1.03 | .776 | 0.99 | 0.96-1.03 | .804 | 1.01 | 0.96-1.06 | .670 |
| WHO Perform | 0-1/2-4 | 2.84 | 1.28-6.29 | .010 | 2.13 | 0.95-4.77 | .065 | 1.68 | 0.66- 4.29 | .275 |
| Sex | M/F | 1.19 | 0.64-2.24 | .582 | 1.12 | 0.63-2.26 | .575 | 0.82 | 0.36-1.89 | .647 |
| Stage | II/III-IV | 0.57 | 0.17-1.91 | .363 | 0.64 | 0.19-2.13 | .464 | 0.54 | 0.12-2.46 | .424 |
| Cytology | Common/blastoid | 1.78 | 0.96-3.30 | .067 | 2.11 | 1.15-3.88 | .015 | 3.43 | 1.74-6.77 | <.001 |
| Ki67 | 0-30/30+ | 1.17 | 0.61-2.23 | .638 | 1.22 | 0.63-2.35 | .558 | 1.44 | 0.66- 3.13 | .361 |
| LDH | Normal/elevated | 1.36 | 0.76-2.41 | .298 | 1.42 | 0.80-2.53 | .228 | 2.34 | 1.17-4.69 | .017 |
| WBC | 0-11/11+ | 1.43 | 0.81-2.54 | .218 | 1.51 | 0.85-2.67 | .161 | 1.62 | 0.83-3.18 | .157 |
| Variable . | Score . | EFS . | PFS . | Survival . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Age | Value | 0.99 | 0.96-1.03 | .776 | 0.99 | 0.96-1.03 | .804 | 1.01 | 0.96-1.06 | .670 |
| WHO Perform | 0-1/2-4 | 2.84 | 1.28-6.29 | .010 | 2.13 | 0.95-4.77 | .065 | 1.68 | 0.66- 4.29 | .275 |
| Sex | M/F | 1.19 | 0.64-2.24 | .582 | 1.12 | 0.63-2.26 | .575 | 0.82 | 0.36-1.89 | .647 |
| Stage | II/III-IV | 0.57 | 0.17-1.91 | .363 | 0.64 | 0.19-2.13 | .464 | 0.54 | 0.12-2.46 | .424 |
| Cytology | Common/blastoid | 1.78 | 0.96-3.30 | .067 | 2.11 | 1.15-3.88 | .015 | 3.43 | 1.74-6.77 | <.001 |
| Ki67 | 0-30/30+ | 1.17 | 0.61-2.23 | .638 | 1.22 | 0.63-2.35 | .558 | 1.44 | 0.66- 3.13 | .361 |
| LDH | Normal/elevated | 1.36 | 0.76-2.41 | .298 | 1.42 | 0.80-2.53 | .228 | 2.34 | 1.17-4.69 | .017 |
| WBC | 0-11/11+ | 1.43 | 0.81-2.54 | .218 | 1.51 | 0.85-2.67 | .161 | 1.62 | 0.83-3.18 | .157 |
CI, confidence interval; F, female; HR, hazard ratio; M, male.
Multivariate analysis of 82 patients with available MRD and PET data who completed transplant
| Variable . | Score . | EFS . | PFS . | Survival . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| MIPI-B | Value | 2.15 | 1.27-3.65 | .004 | 2.29 | 1.33-3.95 | .003 | 3.52 | 1.69- 7.31 | .001 |
| PET response pre-Tx | Pos/neg | 6.00 | 2.37-15.19 | <.001 | 6.82 | 2.63-17.70 | <.001 | 13.79 | 4.07-46.8 | <.0001 |
| CT response pre-Tx | CR/PR | 1.99 | 0.81-4.92 | .134 | 2.20 | 0.87- 5.61 | .097 | 3.66 | 0.89-15.1 | .073 |
| MRD post-Tx | Pos/neg | 4.58 | 1.92-10.89 | .001 | 4.98 | 2.07-12.01 | <.001 | 4.76 | 1.46-15.5 | .001 |
| Variable . | Score . | EFS . | PFS . | Survival . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| MIPI-B | Value | 2.15 | 1.27-3.65 | .004 | 2.29 | 1.33-3.95 | .003 | 3.52 | 1.69- 7.31 | .001 |
| PET response pre-Tx | Pos/neg | 6.00 | 2.37-15.19 | <.001 | 6.82 | 2.63-17.70 | <.001 | 13.79 | 4.07-46.8 | <.0001 |
| CT response pre-Tx | CR/PR | 1.99 | 0.81-4.92 | .134 | 2.20 | 0.87- 5.61 | .097 | 3.66 | 0.89-15.1 | .073 |
| MRD post-Tx | Pos/neg | 4.58 | 1.92-10.89 | .001 | 4.98 | 2.07-12.01 | <.001 | 4.76 | 1.46-15.5 | .001 |
neg, negative; pos, positive; Tx, transplant.
Outcome for patients treated in MCL3 trial compared with the previous MCL2 trial: impact of Zevalin
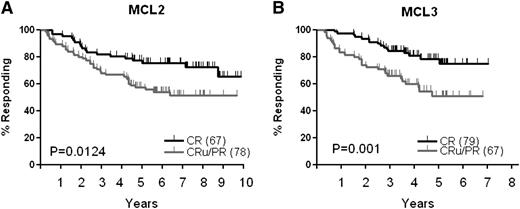
In the MCL2 study, which served as a historical control, patient characteristics were almost identical (Table 1). Unlike for MCL3, there was no Zevalin intensification.5 Response rates achieved after induction therapy in MCL3 and MCL2 were similar (97% and 96%). As shown in Figure 1, survival curves for PFS, EFS, and OS for these 2 studies were superimposable (n = 160). Figure 4 shows the response duration in responders who proceeded to transplant. The duration of response in MCL2 was shorter for pretransplant CRu/PR patients compared with CR patients (Figure 4A). In MCL3, despite the fact that 96% of the patients (64 of 67 patients in CRu or PR) eligible for Zevalin according to protocol did actually receive it, the duration of response for this cohort was still significantly shorter than in the CR group (Figure 4B, P = .001). Twenty-seven of the 67 patients (40%) in CRu/PR have later relapsed or progressed, compared with only 16 of the 79 patients (20%) in CR. Regarding toxicity, we did not observe unexpected severe adverse events related to Z-BEAM/C treatment, and engraftment in terms of recovery time of absolute neutrophil count or platelet count did not differ significantly from those of regular BEAM/C (not shown). An observed incidence of MDS/AML of 4.7% among 64 patients who received Z-BEAM/C was not significantly higher than 1.2% among 82 patients in the BEAM/C alone group (P = .319). One additional case of MDS was observed in a patient who was treated with Zevalin alone outside protocol after failure to harvest stem cells.
Duration of response for patients in CR compared to CRu/PR before transplant. (A) MCL2, (B) MCL3.
Duration of response for patients in CR compared to CRu/PR before transplant. (A) MCL2, (B) MCL3.
Discussion
We have demonstrated that, despite the major improvement in the treatment of younger MCL patients reported for MCL2,5,6 patients who do not achieve a CR after induction treatment have a poorer outcome. To improve this, we here in the MCL3 study offered such patients late intensification with Z-BEAM/C. Compared with the excellent historic control of the Nordic MCL2 patients, we did not succeed in reaching this objective. We confirm that the optimal remission achieved before the transplant, in the present study assessed with CT scanning, PET/CT scanning, and MRD, is the most important factor for the outcome.
Because MCL is highly radiosensitive and expresses surface CD20, targeting radiation directly to the malignant cells with Zevalin is an attractive strategy. Continued exposure to radiation would prevent the tumor cells from DNA damage repair. Wang and colleagues found a response rate of 31% and favorable safety profile in heavily pretreated MCL patients who received Zevalin as single agent.9 As frontline treatment, Zevalin consolidation after 4 cycles of CHOP-rituximab improved response rates in the Eastern Cooperative Oncology Group (ECOG) trial E1499,10 and 131I-tositumomab (Bexxar) followed by CHOP chemotherapy led to a response rate of 86% with 67% CR.17 90Y is a pure β-emitting isotope with energy and path length theoretically yielding a higher cross-fire effect on macroscopic tumors than on single tumor cells. Based on this, we selected patients with residual disease visible on CT scanning before transplant for Zevalin treatment (CRu and PR). Because the treatment was followed by stem cell rescue, Zevalin was not expected to increase hematologic toxicity. Accordingly, Gopal et al treated 16 patients with relapsed MCL with high-dose Bexxar plus chemotherapy followed by autologous stem cell support, and found no unexpected grade III/IV toxicities, and 3-year PFS and OS of 61% and 93%, respectively.18 Standard-dose Zevalin (0.4 mCi/kg) added to high-dose BEAM, similar to our strategy, was first evaluated in a phase 2 trial for patients with relapsed aggressive NHL8 and shown to be well tolerated with median time to WBC and platelet engraftment of 11 and 12 days, and median 2-year PFS and OS of 70% and 89%, respectively. A recent randomized study that compared high-dose BEAM with Z-BEAM in 43 patients with relapsed or refractory aggressive lymphoma11 reported a trend toward a higher rate of mucositis and serious infections in the Z-BEAM arm but no difference in engraftment kinetics. In contrast to our present results in first-line treatment of patients with MCL, the authors showed a statistically significant benefit of Z-BEAM over BEAM in regards to PFS (59% vs 37%) and OS (91% vs 62%) at 2 years. Our historic control study (MCL2) recruited the same number of patients (n = 160) as MCL3 and the patients received similar induction treatment. When comparing the outcome for all patients included in the 2 trials, there were no differences in PFS, EFS, or OS. Despite the treatment of only CRu/PR patients with Z-BEAM/C, the duration of response was still inferior compared with CR patients who received regular BEAM/C conditioning only. Furthermore, a retrospective comparison with the CRu/PR patients in MCL2 did not indicate that late intensification with Zevalin had improved outcome for this patient cohort.
Secondary MDS or AML is a well-known complication of extensive exposure to chemotherapy,19,20 including high-dose therapy with autologous stem cell transplantation where a cumulative incidence of 5% to 10% has been reported.21 There has been concern that Zevalin, especially in heavily pretreated patients, may predispose for MDS/AML. However, Czuczman et al, investigating the incidence of MDS or AML in 746 patients with NHL treated with Zevalin across 5 studies, found 19 (2.5%) MDS/AML cases with a median follow-up of 4.4 years, a rate not higher than expected in a patient population heavily pretreated with cytotoxic agents.22 Likewise, we did not observe a significantly increased incidence of MDS/AML among Z-BEAM/C recipients, compared with BEAM/C recipients.
In the most recent update of the International Working Group response criteria,23 sufficient evidence was not found to recommend PET for staging, response evaluation, or posttherapy surveillance in MCL. Recent retrospective reports, however, suggest a relevance of PET in response assessment.24-26 In our prospective study, a positive PET scan pretransplant was associated with a median PFS of <2 years, significantly shorter than in the PET-negative cohort. The PET-positive PR group is of particular interest: according to the revised response criteria27 , any PET-negative residual mass is compatible with CR. Accordingly, the outcome of our PET-negative patients did not differ irrespective of their response being in PR, CR, or CRu. In multivariate analysis (including MIPI-B, response by CT prior to transplant, and MRD after transplant), a positive PET scan before transplant was shown to be an independent predictor of PFS, EFS, and particularly OS. In retrospective studies,25,26 a positive posttreatment PET scan also predicted early relapse. Our prospective PET-based results strongly suggest a value of this modality in the evaluation of response in MCL. We advocate that patients who do not achieve a negative PET after induction treatment should be considered for additional treatments or alternative strategies instead of going directly on to transplant.
MRD monitoring has been shown in our previous Nordic MCL2 study to be of value to predict prognosis in MCL.6 Furthermore, preemptive rituximab treatment of molecular relapse often converts patients to MRD negative and may delay clinical relapse.28 Pott and colleagues first showed that molecular remission posttransplant in MCL was highly predictive for outcome, with a median PFS of 92 months in the MRD-negative group compared with 21 months in the MRD-positive group (P < .001).29 MRD monitoring has since been performed in the European MCL Network trials and has contributed to a better understanding of quality of remission and risk of relapse.30 In the MCL Younger study, the rate of MRD negativity in the bone marrow rose from 50% to 75% after consolidation with high-dose therapy.31 These results correspond well to our data from MCL3, where 56% of patients were MRD negative before the transplant compared with 86% after. In a multivariate analysis, MRD posttransplant independently contributed to predict PFS, EFS, and OS. Hence, our data are in line with results from the MCL Younger study, suggesting that the tumor reduction achieved by high-dose consolidation contributes to long-term disease-free survival in MCL.
In conclusion, we did not find evidence to support that late intensification with Zevalin added to BEAM/C improves the outcome of patients only in CRu or PR before transplant. A tentative explanation could be that remaining CD20 targets are occupied after multiple doses of rituximab during treatment, hence Zevalin might be competitively unable to attach and exert its action. A positive PET scan prior to transplant and detection of MRD in bone marrow or blood before or after transplant predicted higher rates of relapse and shorter PFS. Because these techniques have independent prognostic values, both may be of importance to guide treatment decisions. For patients with less than a complete response prior to transplant, intervention at that time point may be too late to change the course of disease. High-risk patients can be identified up front by the MIPI and MIPI-B scores, and new strategies are warranted to improve the induction treatment in such patients.
Presented in part at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the medical and nursing staff at all collaborating centers and especially all patients participating in this study. The following members and centers of the Nordic Lymphoma Group contributed with patients to this trial: Peter Meyer, Stavanger University Hospital, Stavanger, Norway; Martin Maisenholder, Tromsø University Hospital, Tromsø, Norway; Bjørn Østenstad, Ullevål University Hospital, Oslo, Norway; Ulf-Henrik Mellqvist, Borås Central Hospital, Borås, Sweden; Jaan Vaart, Skøvde Central Hospital, Skøvde, Sweden; Ingemar Lagerlof, Linkøping University Hospital, Linkøping, Sweden; Martin Erlanson, Norrland University Hospital, Umeå, Sweden; Kristina Arnljots, Malmø University Hospital, Malmø, Sweden; Lars Andreasson, Ørebro University Hospital, Ørebro, Sweden; Sven Erdal, Lidkøping Central Hospital, Lidkøping, Sweden; Eva Mrazek, Karlstad Central Hospital, Karlstad, Sweden; Ilse Christiansen, Ålborg Central Hospital, Ålborg, Denmark; Esa Jantunen, Kuopio University Hospital, Kuopio, Finland; and Outi Kuittinen, Oulu University Hospital, Oulu, Finland. Bayer Schering Pharma contributed with research funding and Zevalin free of charge.
This work was supported by The Nordic Cancer Union and The Norwegian Cancer Society.
Authorship
Contribution: A.K., A. Laurell, E.E., and C.H.G. designed and performed research, collected and analyzed data, and wrote the paper; R.R. and M.J. performed research, collected and analyzed data, and wrote the paper; P.d.N.B. analyzed data and reviewed the manuscript; L.B.P. performed research and reviewed manuscript; and K.G., A. Loft, T.V.B., E.K., P.B.H., U.-M.F., H.N.-E., G.F.L., A.K.L., C.S., M.-L.K.-L., E.R., M.E., J.D., H.B., J.S., K.K.-A., and H.F. collected data and reviewed manuscript.
Conflict-of-interest disclosure: A.K. received research funding from Bayer Schering Pharma for this study. C.H.G. receives research funding and has memberships of advisory boards of GlaxoSmithKline, Celgene, and Roche and is member of the advisory board of Janssen. M.-L.K.-L. receives honoraria from Roche and Mundipharma. The remaining authors declare no competing financial interests.
Correspondence: Arne Kolstad, Department of Oncology, Oslo University Hospital, Norwegian Radium Hospital, PO Box 4590, Nydalen, 0424 Oslo, Norway; e-mail: arnek@ous-hf.no.