In this issue of Blood, Yin et al make the novel discovery that targeting the mucin 1 C-terminal subunit (MUC1-C) oncoprotein reverses resistance to the proteasome inhibitor bortezomib in multiple myeloma cells by triggering depletion of glutathione (GSH) pools and induction of oxidative injury. They also report that these events proceed through a process involving downregulation of the p53-inducible regulator of glycolysis and apoptosis (TIGAR). Specifically, the authors show that MM cells resistant to bortezomib display compensatory upregulation of TIGAR and GSH and that these responses are blocked by a pharmacologic inhibitor of MUC1-C (GO-203), resulting in a marked synergistic increase in reactive oxygen species (ROS) and cell death. One of the more interesting features of this study is that it touches on multiple concepts, including drug resistance, oncogene addiction, oncogenic stress, and inhibition of stress-related pathways in a manner that has very clear translational implications.1
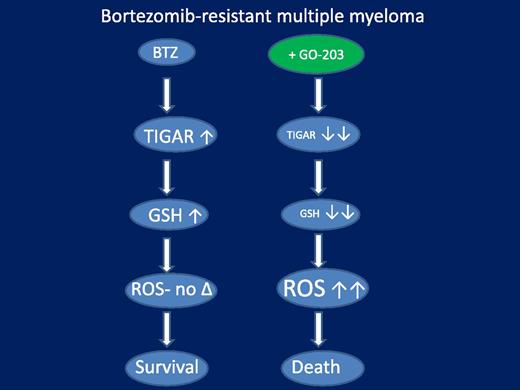
A model of circumvention of bortezomib resistance in bortezomib-resistant multiple myeloma cells by the MUC1 inhibitor GO-203. In this model, resistant cells exhibit an amplified antioxidant response to bortezomib, manifested by upregulation of the glycolytic pathway-regulating protein TIGAR, leading to marked GSH accumulation. The latter sharply reduces the accumulation of toxic ROS species, thereby attenuating cell death. GO-203 effectively blocks TIGAR upregulation and GSH accumulation in bortezomib-treated cells, substantially increasing toxic ROS generation, culminating in the synergistic induction of cells death. Not shown is the induction of ER stress and CHOP upregulation by enhanced oxidative injury in bortezomib-sensitive cells (but not in resistant cells), which may contribute to cell death in the former.
A model of circumvention of bortezomib resistance in bortezomib-resistant multiple myeloma cells by the MUC1 inhibitor GO-203. In this model, resistant cells exhibit an amplified antioxidant response to bortezomib, manifested by upregulation of the glycolytic pathway-regulating protein TIGAR, leading to marked GSH accumulation. The latter sharply reduces the accumulation of toxic ROS species, thereby attenuating cell death. GO-203 effectively blocks TIGAR upregulation and GSH accumulation in bortezomib-treated cells, substantially increasing toxic ROS generation, culminating in the synergistic induction of cells death. Not shown is the induction of ER stress and CHOP upregulation by enhanced oxidative injury in bortezomib-sensitive cells (but not in resistant cells), which may contribute to cell death in the former.
MUC1 is an epithelial heterodimeric protein that is aberrantly expressed in multiple solid tumors, such as breast and colon tumors.2 However, more recently, it has been shown to be expressed and implicated in the survival of several malignant hematopoietic cells, including acute myeloid leukemia and multiple myeloma cells.3,4 Notably, multiple studies have shown that MUC1 is frequently upregulated in multiple myeloma,5 making it an attractive target in this disease. Although MUC1 was initially shown to regulate the activity of prosurvival transcription factors such as nuclear factor (NF)-κB,6 its precise mechanism of action remained to be clearly defined. However, clues emerged, first in solid tumor cells and then in multiple myeloma cells, indicating that MUC1 represents a component of a cellular antioxidant defense program involving TIGAR, a regulator of the pentose phosphate pathway and defender against oxidative stress,7 as well as antioxidant enzymes (eg, reduced NAD phosphate) known to modulate GSH levels.8 Significantly, a peptide inhibitor of MUC1 (GO-203) was developed, which effectively downregulates and disables this system, triggering multiple myeloma cell death and/or necrosis.8
The question naturally arose whether, and by what mechanism, disabling this defensive oxidative stress system might modify the response of bortezomib-resistant myeloma cells to bortezomib. Despite the impressive activity of proteasome inhibitors such as bortezomib and carfilzomib in myeloma, their primary mode of action remains uncertain and has been variably ascribed to NF-κB inactivation, induction of proteotoxic stress, and accumulation of proapoptotic proteins, among numerous others. Analogously, the basis for bortezomib resistance is unclear, and although preclinical studies have implicated multidrug resistance efflux mechanisms or proteasome subunit mutations, the relevance of these factors for resistance in patients remains to be established.4 Interestingly, a link between proteasome inhibitor actions and induction of ROS/oxidative injury in neoplastic cells has long been recognized.9 Consistent with this observation, it is noteworthy that Lin et al found that bortezomib-resistant myeloma cells exhibited amplified activation of antioxidant defenses through the MUC1-TIGAR-GSH axis in response to bortezomib and that blocking this response, for example, by GO-203, significantly increased oxidative injury (eg, superoxide and hydrogen peroxide generation) and cell death. A model summarizing these points is illustrated in the figure. Notably, in bortezomib-sensitive cells, oxidative injury also stimulated lethal components of the unfolded-protein response (UPR), for example, CCAAT/enhancer binding protein homologous protein (CHOP) upregulation, potentially contributing to cell death. The UPR represents a cellular response to the accumulation of mal-folded proteins in the endoplasmic reticulum (endoplasmic reticulum stress), and although it initially plays a cytoprotective role, if unresolved it can transform into a proapoptotic one. Together, these findings are consistent with a model in which MUC1 and its downstream effectors protect myeloma cells from both intrinsic and bortezomib-induced oxidative and proteotoxic stress, and that interference with this defensive mechanism renders myeloma cells particularly sensitive to noxious stimuli. Such a model is consistent with the strategy of targeting so-called “orthogonal” pathways, that is, those that are not directly responsible for neoplastic transformation, such as those related to oncoproteins with driver mutations, but instead are required to protect neoplastic cells from resulting oncogenic stresses.10
These findings raise multiple questions that could stimulate a variety of future investigations and identification of therapeutic opportunities. For, example, it will be interesting to determine whether the capacity of agents like GO-203 to disrupt the MUC1 pathway and overcome bortezomib resistance is restricted to bortezomib or could be extended to newer-generation proteasome inhibitors such as carfilzomib, which demonstrates intrinsic activity in some bortezomib-resistant cells. In this context, it will also be interesting to determine whether these findings could also be extended to other inhibitors of the ubiquitin-proteasome system, for example, neural precursor cell expressed, developmentally downregulated 8 or deubiquitinase inhibitors, which may also trigger oxidative or proteotoxic stress. Significantly, some of these agents have now entered the clinical arena. A question also arises of whether this strategy might be effective in other hematologic malignancies in which proteasome inhibitors have established (eg, mantle cell lymphoma) or potential (eg, activated B-cell-diffuse large B-cell lymphoma) therapeutic roles. In particular, extension of these findings to solid tumors, which for unknown reasons remain largely unresponsive to proteasome inhibitors, would obviously have major implications. Additionally, it will be critical to determine whether similar resistance mechanisms are operative in cells obtained from patients exposed to proteasome inhibitors in vivo, and if so, whether circumvention of resistance ensues. Finally, it will be important to identify the mechanism(s) by which this strategy selectively enhances proteasome inhibitor lethality in neoplastic compared with normal cells, thereby increasing the therapeutic index. Given ongoing efforts to develop GO-203 for clinical use, answers to several of these questions, and potentially validation of this strategy, are likely to be addressed in the not too distant future.
Conflict-of-interest disclosure: The author declares no competing financial interests.