In this issue of Blood, Bénézech and colleagues demonstrate a role for platelets beyond fetal development, to maintaining integrity of the adult lymphatic system.1
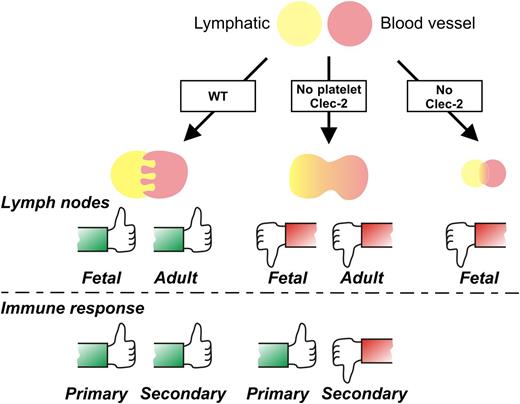
Megakaryocyte/platelet CLEC-2 expression is required for a fully functional lymphatic system, according to a new study by Bénézech and colleagues.1 The thumbs-up symbol indicates like wild-type (WT); thumbs down indicates abnormal lymph node development and blood-lymph mixing and/or immune impairment. (A more detailed model is depicted in supplemental Figure 5 of the article by Bénézech et al,1 available on the Blood Web site.)
Megakaryocyte/platelet CLEC-2 expression is required for a fully functional lymphatic system, according to a new study by Bénézech and colleagues.1 The thumbs-up symbol indicates like wild-type (WT); thumbs down indicates abnormal lymph node development and blood-lymph mixing and/or immune impairment. (A more detailed model is depicted in supplemental Figure 5 of the article by Bénézech et al,1 available on the Blood Web site.)
Lympha, the ancient Roman deity with the portfolio of “fresh water,” herself could hardly be more surprised at the profusion of recent studies showing a crucial role for blood platelets in regulating her eponymous circulatory system. Water in the body cycles between blood, tissue fluid, and lymph compartments, providing a medium for proteins, fats, carbohydrates, and cells. Accumulation of excess fluid in tissues due to heart failure or other causes leads to swelling of tissue or limbs and the painful condition of dropsy (edema). From the fetus to the grave, maintaining healthy vasculature and lymphatic networks is essential for blood supply, wound healing, fighting infection, inflammation, and the immune response. Lymph fluid drains from intercellular spaces of tissues through lymphatic vessels to return to the blood. Lymph nodes in the lymphatic circulation are organized confluences of lymphatic endothelial cells which separate lymph from blood, and provide a barrier regulating passage between lymph and blood.2-5 Lymphocytes routinely enter lymph nodes via high endothelial venules for immune surveillance, but remarkably without excessive blood mixing or leakage. The lymphatic system may also transport bacteria or metastatic tumor cells. Recent research has revealed a role for blood platelets in regulating the lymphatic circulation and immune response, and, importantly from a therapeutic perspective, have identified some of the key molecules involved: C-type lectinlike receptor-2 (CLEC-2) on platelets2-5 and its binding partner, podoplanin.6
In the bloodstream, platelets play a key role in primary hemostasis, responding to injury or endothelial perturbation by becoming rapidly activated, secreting autocrine platelet activators (adenosine 5′-diphosphate, thromboxane), switching on platelet integrin αIIbβ3 that mediates platelet aggregation, and releasing proinflammatory and procoagulant mediators regulating the localized recruitment of leukocytes. The hemostatic response occurs within seconds to minutes to prevent blood loss, promote wound healing, and stimulate the inflammatory response. Primary platelet-specific receptor complexes, glycoprotein Ib-IX-V (GP Ib-IX-V), and GPVI/FcRγ play an essential role in cross-talk between these vascular systems by binding adhesive ligands, mainly von Willebrand factor and collagen, coagulation factors, and/or counterreceptors on endothelial cells (P-selectin) and leukocytes (integrin αMβ2).7 Ligand binding to GPVI activates immunoreceptor tyrosine-based activation motif (ITAM)–dependent signaling via an ITAM in the associated Fc γ-chain, thereby activating the Syk kinase signaling cascade involving downstream activation of phospholipase Cγ and Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76).4,5 The ITAM/Syk pathway is also intimately associated with CLEC-2 function.
CLEC-2 (gene name CLEC1B or Clec1b), originally discovered as a platelet receptor for the snake toxin rhodocytin, contains an extracellular C-type lectin-like domain and a hemi-ITAM motif within its cytoplasmic domain, and signals via the ITAM-based Syk-dependent signaling pathway.4,5 Knockout of CLEC-2 in mice has adverse consequences on embryonic development, whereas postnatal deficiency in platelets by conditional knockout or antibody-based depletion results in no or minimal impact on tail bleeding, but a more pronounced effect on arterial thrombosis.4,5,8 There is no readily identifiable source of podoplanin in blood vessel endothelial cells or the subendothelial matrix,4 suggesting other CLEC-2 ligand(s) or a synergistic contribution to platelet activation. In this regard, other studies have shown that ITAM-related signals by GPVI/FcRγ and CLEC-2 are, in part, functionally redundant in mice; that is, selective deletion of either receptor has markedly less severe bleeding phenotype than combined deficiency of both receptors8 (human, but not mouse platelets, contain a third ITAM-based immunoreceptor, FcγRIIa). As discussed further below, these pathways are likely to be of crucial importance for CLEC-2 function in lymphatic regulation.
The physiological ligand identified for CLEC-2 is podoplanin, a transmembrane mucin-like protein widely expressed on different cell types.6 This protein is also expressed on tumor cells where platelet interactions could be involved in metastasis.2-6 But it is podoplanin on lymphatic vasculature where interactions with platelet CLEC-2 control the integrity of this system in fetal and adult mice. Bénézech and colleagues used mouse models with CLEC-2 deficiency or megakaryocyte/platelet-selective CLEC-2 deficiency to identify 2 distinct roles for this receptor in either lymphatic formation in the embryo or maintenance of functional lymph nodes in adults.1 Defects were defined by attenuated lymphatic endothelial cell proliferation and malformed lymphatic vasculature in constitutive knockouts, while megakaryocyte/platelet knockouts displayed increased blood mixing with lymph nodes, with minimal effect on primary immune response, but compromised recruitment of lymphocytes regulating secondary acquired immunity (see figure).
Earlier studies have shown that both Syk- and SLP-76–deficient mice also display abnormal lymphoidal development,5 suggesting that CLEC-2 downstream signals following podoplanin engagement are critical for fulfilling these lymphatic-related functions. In contemporary studies, Herzog et al address related questions using different models, but show defects in lymphocyte transition at lymph nodes in postnatal mice lacking podoplanin or with antibody-depleted platelet CLEC-2, and bleeding associated with immunization, which is rescued by blockade of lymphocyte homing or by transfusion of CLEC-2–expressing platelets into the mice.9 In further mechanistic insights from this study, some of the effects of CLEC-2/podoplanin deficiency were related to reduced expression of the cell-cell adhesion receptor, VE-cadherin on high endothelial venules, and CLEC-2–dependent release of platelet sphingosine-1-phosphate, an angiogenic factor which upregulates expression of VE-cadherin.9 Adding to the array of potential targets, glycosylation modifications are also implicated in both CLEC-2–podoplanin interactions as well as lymphatic development.6 Evaluating different immune models and inhibitors against these various targets requires further investigation. As found for murine inflammation models,10 correlating all of these findings with more complex human systems is also a significant future challenge.
In summary, recent1,9 and earlier studies reviewed elsewhere2-5 clearly identify a new role for CLEC-2/podoplanin in fetal/adult development/maintenance of the mouse lymphatic system. Further research is needed to take advantage of these findings: experimentally, new intravital approaches in mice could help understand blood-lymph system interactions in real time. Clinically, examining these exciting discoveries in the complex human system in relation to (1) thrombocytopenia, platelet activation status, and hyperreactivity associated with elevated atherothrombotic risk, (2) effect of other platelet ITAM-based receptors (GPVI/FcRγ, FcγRIIa), or (3) consequences of antiplatelet drugs on CLEC-2 signaling and function promises to provide new diagnostic or treatment options for immunologic or nonimmunologic defects involving human blood-lymphatic systems.
Conflict-of-interest disclosure: The authors declare no competing financial interests.