In this issue of Blood, Walsby et al describe a novel in vitro model to study chronic lymphocytic leukemia (CLL) trafficking under flow, reporting the regulation of key homing molecules by shear force.1
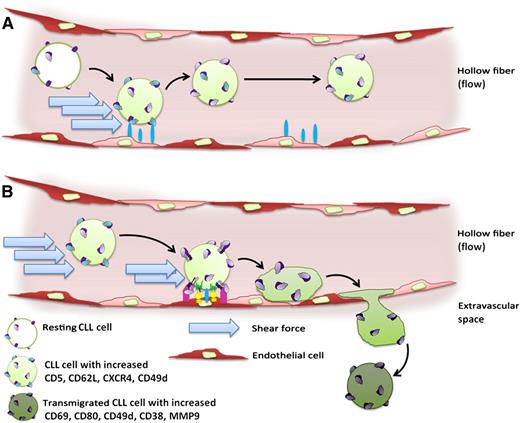
While circulating in the endothelium-lined hollow fiber system, (A) CLL cells experience strong shear forces and short-lived contacts with endothelial cells, resulting in their increased expression of CD5, CD62L, CXCR4, and CD49d. (B) Compared with the circulating bulk, the collected transmigrated subpopulation of CLL cells is characterized by increased expression of CD69, CD80, CD49d, CD38, and MMP9.
While circulating in the endothelium-lined hollow fiber system, (A) CLL cells experience strong shear forces and short-lived contacts with endothelial cells, resulting in their increased expression of CD5, CD62L, CXCR4, and CD49d. (B) Compared with the circulating bulk, the collected transmigrated subpopulation of CLL cells is characterized by increased expression of CD69, CD80, CD49d, CD38, and MMP9.
It is increasingly recognized that the cellular biology underlying CLL pathophysiology is dynamic, with mostly nonproliferative CLL cells in the peripheral blood but substantial rates of proliferating CLL cells in lymph nodes and other lymphoid organs.2 This “awakening” of CLL cells by supporting lymphoid-derived factors and accessory cells requires a continuous CLL cell recirculation between the blood and lymphoid organs. The pathophysiological importance of the dynamic lymphoid positioning of CLL cells demands a different mode of therapeutic intervention, which is impressively reflected in the current clinical success of novel agents altering CLL cell localization.3
To exit the peripheral blood and re-enter lymphoid organs, a process called homing, CLL cells likely use mechanisms similar to those of normal immune cell trafficking, involving l-selectin (CD62L)-, chemokine receptor (CXCR4)-, and integrin (very late antigen-4, CD49d/CD29)-dependent steps. Extravasation is based on a coordinated and interdependent sequence of these adhesive cues that are highly influenced by the fluid shear stress exerted on the cells in the circulation. Although the direct regulating role of shear force on adhesion molecule function is well known in the immunological context, most in vitro migration systems used for CLL and endothelial research still ignore this factor.
To establish an in vitro transendothelial migration system that allows flow to exert shear stress on CLL and endothelial cells over several days, Walsby et al used an adapted hollow fiber bioreactor with the interior of the fibers lined with human umbilical vein endothelial cells or cells of a microvascular endothelial cell line (HMEC-1).1 CLL cells were subjected to this system and could be recollected at different ports, which allowed the ability to distinguish between circulating (nontransmigrated) and transmigrated CLL cells. The authors observed that the presence of shear force caused an alteration of the phenotype of both the endothelial and CLL cells. As a result of the experienced shear force and the transient (weak) interactions with endothelial cells, CLL cells increased their expression of CD5, CD62L, CXCR4, and CD49d (panel A). Moreover, the subpopulation of CLL cells that was able to undergo transmigration displayed a significantly increased expression of the early activation marker CD69, the costimulatory molecule CD80, and CD49d, CD38, and MMP9 (panel B), which play well-known roles in CLL homing, survival, and proliferation.4-6 It remains to be elucidated whether this observation is caused by a preferential transendothelial migration of a CLL subpopulation with higher expression of these molecules or whether the combined exertion of shear stress and transient contact with endothelial cells can induce increased expression of CD49, CD38, and MMP9 on CLL cells. Notably, the recovered transmigrated subpopulation was very small (1.37 ± 2.14% of injected cells after 48 hours), but despite this fact, transmigration was an active, reproducible, and specific process and could be strongly antagonized by anti-CD49d antibodies. Further, transmigration rates were directly correlated to the CD49d expression of the cells. These findings are consistent with the previously reported reduced transendothelial migratory capacity of CLL cells7-9 and the key role of CD49d in CLL homing to lymphoid organs.4,10 All findings together may suggest that the recirculation of even a minor fraction of CLL cells, enriched in relevant homing factors, might be sufficient to drive the pathophysiology of the disease.
From a more translational perspective, the novel system of Walsby et al1 is also a step forward toward more physiological in vitro models to study the effects of novel drugs specifically on CLL circulation and transmigration, which are the most relevant components of CLL pathophysiology.
Conflict-of-interest disclosure: The author declares no competing financial interests.