Key Points
Factor IX glutamyl carboxylation in engineered HEK 293 cells recapitulates in vivo anticoagulant inhibition of vitamin K cycle activity.
Warfarin metabolite structure-activity analysis on vitamin K cycle antagonism determines their contributions to in vivo anticoagulation.
Abstract
Warfarin and other 4-hydroxycoumarins inhibit vitamin K epoxide reductase (VKOR) by depleting reduced vitamin K that is required for posttranslational modification of vitamin K–dependent clotting factors. In vitro prediction of the in vivo potency of vitamin K antagonists is complicated by the complex multicomponent nature of the vitamin K cycle. Here we describe a sensitive assay that enables quantitative analysis of γ-glutamyl carboxylation and its antagonism in live cells. We engineered a human embryonic kidney (HEK) 293–derived cell line (HEK 293-C3) to express a chimeric protein (F9CH) comprising the Gla domain of factor IX fused to the transmembrane and cytoplasmic regions of proline-rich Gla protein 2. Maximal γ-glutamyl carboxylation of F9CH required vitamin K supplementation, and was dose-dependently inhibited by racemic warfarin at a physiologically relevant concentration. Cellular γ-glutamyl carboxylation also exhibited differential VKOR inhibition by warfarin enantiomers (S > R) consistent with their in vivo potencies. We further analyzed the structure-activity relationship for inhibition of γ-glutamyl carboxylation by warfarin metabolites, observing tolerance to phenolic substitution at the C-5 and especially C-6, but not C-7 or C-8, positions on the 4-hydroxycoumarin nucleus. After correction for in vivo concentration and protein binding, 10-hydroxywarfarin and warfarin alcohols were predicted to be the most potent inhibitory metabolites in vivo.
Introduction
Vitamin K antagonists, such as warfarin, remain a mainstay of oral anticoagulant therapy. However, patient response to these drugs is highly variable, and much effort has been expended to identify through genomic approaches the factors that contribute to this variability.1,2 Consideration of genetic and clinical variables has led to the development of algorithms that account for as much as 50% of the variance in warfarin dose.3,4 However, there is interest in identifying approaches that can improve warfarin therapy, particularly during long-term treatment when changes in vitamin K intake and/or therapy with concomitantly administered medications may alter otherwise stabilized warfarin pharmacokinetics and/or pharmacodynamics. In vitro evaluation of the former is relatively easy, given that it is well established that CYP2C9 and CYP3A4 are the major drug-metabolizing enzymes that control the metabolic clearance of (S)- and (R)-warfarin, respectively.5 In contrast, in vitro approaches to studying warfarin pharmacodynamics require a facile, quantitative reporter of vitamin K cycle activity.
Vitamin K is the essential cofactor for the key reaction of the vitamin K cycle, specifically the γ-glutamyl carboxylase (GGCX)-mediated carboxylation of Glu residues on at least 17 Gla proteins identified in vertebrates that are critical for blood clotting, bone health, inhibition of soft-tissue calcification, and cell growth regulation.6 The process of γ-glutamyl carboxylation also generates vitamin K epoxide that must be reduced back to vitamin K by vitamin K epoxide reductase (VKOR) to maintain vitamin K cycle activity.7 Vitamin K antagonists, such as warfarin, exert their therapeutic effects by inhibiting VKOR, resulting in impaired recycling of vitamin K and a concomitant decrease in the generation of appropriately posttranslationally modified clotting factors II (prothrombin), VII, IX, and X.8
Despite over 60 years of clinical use of warfarin and the identification of the genes encoding GGCX9 and VKOR,10,11 much remains to be learned about the function of the vitamin K cycle, the identities and roles of ancillary cellular factors that support its function, the detailed molecular mechanism whereby warfarin exerts its anticoagulant effects, and the nature of complex interactions with xenobiotics that impact warfarin therapy. Both VKOR and GGCX are integral transmembrane proteins, a feature that has necessitated their functional characterization in detergent-solubilized or microsomal preparations. This complicated efforts to clarify the nature of their interaction with one another and has also required the use of dithiothreitol (DTT) rather than the endogenous reductant of VKOR. Although some drug-drug and drug-gene interactions involving warfarin are easily understood on the basis of disruptions in CYP2C9 activity, which can be readily evaluated in vitro,5 direct effects of modulators of vitamin K cycle enzymes have proven resistant to study owing to the requirement for a membrane context and potential interplay between GGCX and VKOR.
In an effort to circumvent these limitations, we have developed a simple cell-based assay for the facile quantitative measurement of vitamin K cycle activity. This method relies on the flow cytometric detection of the γ-glutamyl-carboxylated and calcium-bound conformation of the human factor IX Gla domain, which is presented on the surface of living cells by replacement of the Gla domain of human proline-rich Gla protein 2, a vitamin K–dependent integral membrane protein.12 After validating this assay system by confirming its dependence on supplementation with exogenous vitamin K and observing its expected differential responsiveness to warfarin enantiomers, we applied this method to determine the intrinsic biological activities of warfarin metabolites with respect to VKOR antagonism.
Materials and methods
Chemical reagents
Vitamin K1 was purchased from Hospira. Racemic warfarin was purchased from Sigma-Aldrich. Warfarin enantiomers, the warfarin alcohols, and 4′, 5-, 6-, 7-, 8- and 10-hydroxywarfarin metabolites (gifts from the late Professor William Trager [University of Washington, Seattle, WA]) were obtained as previously described.13,14
Cell culture
Human embryonic kidney (HEK) 293 Tet-On (Clontech) and HEK 293-C3 cells were cultured under a humidified 5% CO2 atmosphere in regular growth medium (RGM) consisting of 90% Dulbecco's modified Eagle medium (DMEM; Gibco), 10% Tet system approved fetal bovine serum (Clontech), 4mM l-glutamine, 200 μg/mL G418, 100 U/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate. Adherent cells were cultured on plates coated with 50 μg/mL type I rat tail collagen (Becton Dickinson) and routinely passaged at 70% to 80% confluence following dissociation with 0.25% trypsin/EDTA (Invitrogen).
Expression of chimeric F9CH
HEK 293-C3 cells were detached from stock plates with trypsin/EDTA and seeded on collagen-coated 6-well plates at a density of 1.0 × 104 cells per well in RGM supplemented with 200 μg/mL hygromycin, 50 μM d-biotin, and 50nM vitamin K1. Doxycycline was added at 2 μg/mL 24 hours after plating to induce expression of F9CH. Forty-eight hours after doxycycline addition, cells were detached by incubating in StemPro Accutase (Invitrogen) at 37°C for 5 minutes, diluted 10-fold into a buffer consisting of 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 150 mM NaCl, 2mM EDTA, and 1% weight to volume ratio bovine serum albumin, and collected by gentle centrifugation. Cells were washed once in staining buffer (SB) consisting of 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150 mM NaCl, 5 mM CaCl2, and 1% bovine serum albumin, centrifuged, and resuspended in SB containing 5 μg/mL anti-Factor-IX Gla domain antibody GMA-001 (Green Mountain Antibodies) conjugated to allophycocyanin (APC) with Lynx Rapid APC reagent (AbD Serotec). Cells were immunostained for 20 minutes at 4°C, diluted 10-fold with SB, collected by gentle centrifugation, and subjected to dual-color fluorescence-activated cell sorting (FACS).
Flow cytometric analysis
HEK 293 Tet-On and HEK 293-C3 cells were cultured and immunostained as described in the previous paragraph. For each sample, red fluorescent protein from Discosoma sp. (DsRed) and APC fluorescence of 104 cells was measured using a FACSCanto II flow cytometer (Becton Dickinson), with excitation/emission wavelengths of 554/585 nm for DsRed and 633/660 nm for APC. For each sample, median DsRed and APC fluorescence were determined with FlowJo analysis software (TreeStar Inc.). DsRed and APC fluorescence in HEK 293-TO cells (lacking DsRed and F9CH expression) that had been stained with the APC-conjugated GMA-001 antibody was subtracted from fluorescence measurements in HEK 293-C3 cells to account for background. Median DsRed and APC fluorescence for each sample was divided by the equivalent measurements in the samples with the greatest fluorescence and expressed as a percentage of maximum median fluorescence for a given experiment. The difference between maximum and minimum median APC fluorescence in a typical experiment was 10- to 15-fold.
Determination of fraction unbound for warfarin and its circulating metabolites
Protein binding of warfarin as well as its circulating metabolites was measured in both human plasma and RGM by ultracentrifugation to determine fraction unbound in plasma (fuPlasma) and fraction unbound in RGM (fuMedia). Stock solutions (100× concentrated in methanol) containing warfarin or its phenol and alcohol metabolites were spiked into samples of either RGM or blank human plasma, generating final concentrations of metabolites ranging from 0.5 μM to 2.0 μM. Protein binding for warfarin and most metabolites was measured together in samples spiked from a mixed stock solution, while 5-hydroxywarfarin protein binding was measured separately. Aliquots (200 μL) were centrifuged at 435 000g at 37°C for 2 hours or incubated, without centrifugation, at 37°C for 2 hours. After ultracentrifugation/incubation, 50-μL aliquots were removed, precipitated in 50 μL of MeCN and 20 mL of 10% HCl, and spiked with an internal standard mix of 4′-hydroxywarfarin-d4, 6-hydroxywarfarin-d5, 7-hydroxywarfarin-d5, and 8-hydroxywarfarin-d5. Samples were vortexed, centrifuged (7800g, 10 minutes), and analyzed by liquid chromatography-mass spectrometry. Standard curves were determined for the parent drug and each metabolite over a range from 0.005 μM to 2 μM in both human plasma and RGM. The RGM (fuMedia) and plasma (fuPlasma) free drug fractions were calculated as the ratio of the unbound concentration (determined from triplicate centrifuged samples) to total concentration (determined from triplicate uncentrifuged samples), and are presented as average values, with standard deviations, determined from 3 different spiked concentrations for each metabolite (0.5 μM, 1.0 μM, and 2.0 μM).
Determination of in vitro and in vivo inhibitory potential of warfarin and its circulating metabolites
[I]/IC50 was determined by dividing the maximum published circulating drug or metabolite concentration [I] in human plasma15-17 by the half maximal inhibitory ligand concentration (IC50) in vitro for inhibition of F9CH γ-carboxylation. [I]u/Kiu was calculated as the ratio of unbound plasma drug or metabolite concentration [I]u (where [I]u = [I] × fuPlasma) to the IC50 determined in vitro for inhibition of F9CH γ-carboxylation corrected for drug or metabolite binding to RGM, Kiu, (where Kiu = IC50 × fuMedia).
Ethics statement
Experiments in this study were performed on liver tissue from deceased, anonymous individuals; the study is therefore not considered to involve “human subjects.” Samples were collected with approval of an institutional review board and the University of Washington Institutional Review Board approved their use for this study. This study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical analysis was performed using Prism statistical software (GraphPad Software). IC50 values were determined by nonlinear regression (curve fit). Comparisons between groups were made using an unpaired Student t test, with P < .05 considered significant.
Results
Factor IX Gla/DsRed expression system
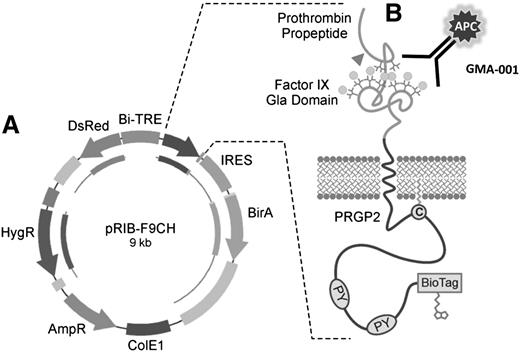
The clonal cell line HEK 293-C3 was generated by stable transfection of HEK 293-TO cells with plasmid pRIB-F9CH (Figure 1A). This plasmid directs the doxycycline-inducible expression of a fusion protein consisting of the human prothrombin pre-pro-peptide, the Gla domain of human factor IX, the transmembrane and cytoplasmic domains of proline-rich Gla protein 2, and an Escherichia coli biotin ligase acceptor site (BioTag). This plasmid simultaneously drives expression of DsRed in the cytoplasm and the chimeric F9CH reporter in the plasma membrane oriented such that the factor IX Gla domain is exposed to the extracellular space. This enables simultaneous flow cytometric monitoring of γ-glutamyl carboxylation of the factor IX Gla domain, by using a Gla-dependent and conformation-specific anti-factor IX antibody (Figure 1B) and DsRed expression for normalization.
Factor-IX Gla/Ds Red expression system. (A) Vector with doxycycline-inducible bidirectional promoter directing expression of cytoplasmic DsRed to assess induction, and chimeric factor-IX Gla domain-containing protein (F9CH) to measure carboxylation activity. Tet-On cells containing this vector are referred to as the “C3” cell line. (B) Chimeric factor-IX Gla protein contains prothrombin signal and pre-pro-peptide directing γ-carboxylation of the factor-IX Gla domain. APC-labeled antibody (GMA-001) specifically binds the γ-carboxylated factor-IX Gla domain.
Factor-IX Gla/Ds Red expression system. (A) Vector with doxycycline-inducible bidirectional promoter directing expression of cytoplasmic DsRed to assess induction, and chimeric factor-IX Gla domain-containing protein (F9CH) to measure carboxylation activity. Tet-On cells containing this vector are referred to as the “C3” cell line. (B) Chimeric factor-IX Gla protein contains prothrombin signal and pre-pro-peptide directing γ-carboxylation of the factor-IX Gla domain. APC-labeled antibody (GMA-001) specifically binds the γ-carboxylated factor-IX Gla domain.
Expression of genes associated with vitamin K and warfarin metabolism
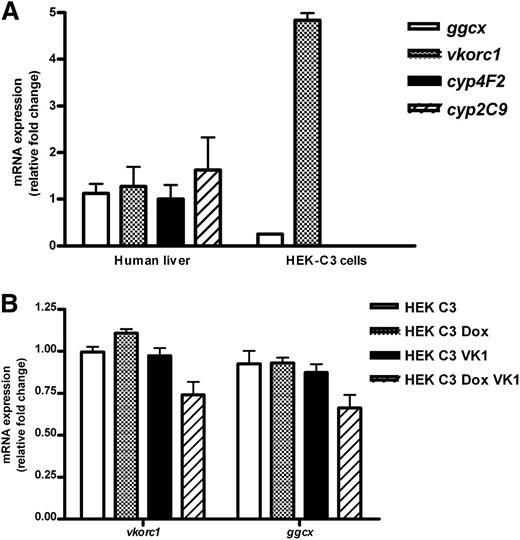
The expression levels of genes critical for the vitamin K cycle, vitamin K metabolism, and warfarin metabolism were measured in the HEK 293-C3 cell line to evaluate its suitability as a sensitive system for measuring vitamin K–dependent γ-glutamyl carboxylation. Consistent with previous reports of endogenous γ-carboxylation activity in HEK-293 cells,18 HEK 293-C3 cells expressed both vkorc1 and ggcx. We genotyped our HEK 293-C3 cells for the vkorc1 −1639 G>A polymorphism known to reduce expression of VKORC1 in humans, and determined they are heterozygous for this allele. We compared expression of genes involved in vitamin K and warfarin metabolism in HEK-293 C3 cells with that in human livers (n = 6) previously genotyped as heterozygous for the 1639 G/A allele of vkorc1.19 HEK 293-C3 cells have significantly lower expression of ggcx but higher expression of vkorc1 than the human livers assayed (Figure 2A). In contrast, we were unable to detect in HEK 293-C3 cells the expression of CYP2C9 or CYP4F2 (Figure 2A), or that of any other drug-metabolizing P450 genes known to be involved in the metabolism of either warfarin enantiomer or vitamin K (not shown). The expression of ggcx and vkorc1 was not significantly affected by addition of vitamin K1 or by the induction of expression of the F9CH reporter by doxycycline (Figure 2B).
Expression of vitamin K–associated gene expression in human liver and HEK 293-C3 cells and effects of vitamin K supplementation and doxycycline induction on gene expression in HEK-C3 cells. (A) Gene expression in subconfluent, nondoxycycline-induced, nonvitamin K1–supplemented HEK 293-C3 cells was normalized to its own 18S ribosomal RNA expression and relative fold change determined by comparing to expression in pooled human liver complementary DNA (n = 6), which was set as 1.0. Expression of cytochrome P450’s 2C9 and 4F2 was not detectable in HEK-C3 cells. *P < .5 compared with human liver. (B) HEK-293 C3 cells were plated in regular growth media, with vitamin K1 (VK1) or without supplementation with 50 nM vitamin K1. Twenty-four hours after plating, expression of the F9CH plasmid was induced in some samples by addition of 200 μg/mL doxycycline (Dox). All cells were harvested 48 hours after plating. Gene expression in each sample was normalized to its own 18S rRNA expression and relative fold change determined by comparing to expression in pooled, nondoxycycline-induced, nonvitamin K1–supplemented HEK 293-C3 cDNA (n = 3), which was set as 1.0. Expression of cytochrome P450’s 2C9 and 4F2 was not detectable in HEK-C3 cells. mRNA, messenger RNA.
Expression of vitamin K–associated gene expression in human liver and HEK 293-C3 cells and effects of vitamin K supplementation and doxycycline induction on gene expression in HEK-C3 cells. (A) Gene expression in subconfluent, nondoxycycline-induced, nonvitamin K1–supplemented HEK 293-C3 cells was normalized to its own 18S ribosomal RNA expression and relative fold change determined by comparing to expression in pooled human liver complementary DNA (n = 6), which was set as 1.0. Expression of cytochrome P450’s 2C9 and 4F2 was not detectable in HEK-C3 cells. *P < .5 compared with human liver. (B) HEK-293 C3 cells were plated in regular growth media, with vitamin K1 (VK1) or without supplementation with 50 nM vitamin K1. Twenty-four hours after plating, expression of the F9CH plasmid was induced in some samples by addition of 200 μg/mL doxycycline (Dox). All cells were harvested 48 hours after plating. Gene expression in each sample was normalized to its own 18S rRNA expression and relative fold change determined by comparing to expression in pooled, nondoxycycline-induced, nonvitamin K1–supplemented HEK 293-C3 cDNA (n = 3), which was set as 1.0. Expression of cytochrome P450’s 2C9 and 4F2 was not detectable in HEK-C3 cells. mRNA, messenger RNA.
Vitamin K dependence and warfarin inhibition of F9CH γ-carboxylation
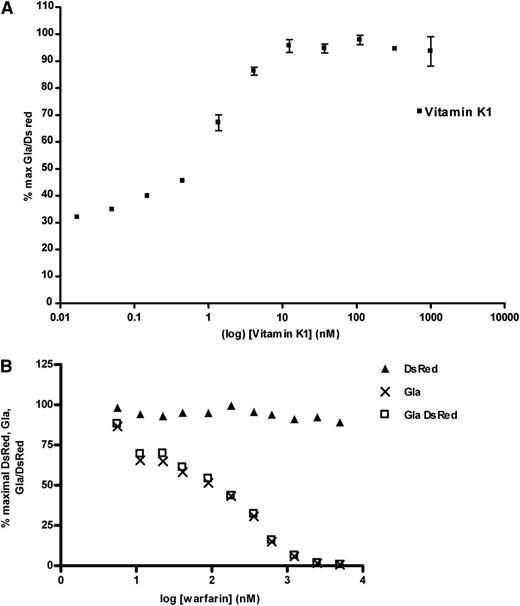
We examined the vitamin K dependence of F9CH γ-glutamyl carboxylation in HEK 293-C3 cells and its sensitivity to warfarin inhibition. Supplementation of doxycycline-treated HEK 293-C3 cells with vitamin K1 from 0.01 nM to 1000 nM increased the γ-glutamyl-carboxylated F9CH antigen in a dose-dependent manner (Figure 3A). Maximal γ-glutamyl carboxylation was observed at a vitamin K1 concentration of ∼10 nM, which is similar to recently reported plasma vitamin K1 concentrations in humans by Shea et al of 2.0 nM to 3.8 nM20 or Kamao et al of 2.8 nM.21 The observation that γ-glutamyl carboxylation proceeded in the absence of exogenous vitamin K suggests the presence of an endogenous vitamin K vitamer in a component of the cell medium. We were, however, unable to detect vitamin K1 in the fetal bovine serum. Vitamin K1–supported F9CH carboxylation in HEK 293-C3 cells was inhibited in a dose-dependent manner by the addition of racemic warfarin, whereas DsRed expression was unaffected (Figure 3B). Vitamin K1-supported F9CH carboxylation in HEK 293-C3 cells was inhibited in a dose dependent manner by the addition of racemic warfarin, whereas DsRed expression was unaffected (Figure 3B; supplemental Figure 1).
F9CH γ-carboxylation in HEK-C3 cells is vitamin K dependent and inhibited by VKOR antagonists. (A) Doxycycline-induced HEK-C3 cells were supplemented with vitamin K1 and assayed by flow cytometry for γ-carboxylated F9CH and DsRed as described. F9CH Gla and DsRed signals were calculated as percent of maximal, and corrected for background in naïve HEK-Tet-ON parental cell line immunostained for γ-carboxylated F9CH. Percent maximal Gla/DsRed (▪) is plotted. (B) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of R,S-warfarin were assayed by flow cytometry for γ-carboxylated F9CH (and DsRed fluorescence). F9CH Gla and DsRed signals were calculated as percent of maximal, and corrected for background in naive HEK-Tet-ON parental cell line immunostained for γ-carboxylated F9CH.
F9CH γ-carboxylation in HEK-C3 cells is vitamin K dependent and inhibited by VKOR antagonists. (A) Doxycycline-induced HEK-C3 cells were supplemented with vitamin K1 and assayed by flow cytometry for γ-carboxylated F9CH and DsRed as described. F9CH Gla and DsRed signals were calculated as percent of maximal, and corrected for background in naïve HEK-Tet-ON parental cell line immunostained for γ-carboxylated F9CH. Percent maximal Gla/DsRed (▪) is plotted. (B) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of R,S-warfarin were assayed by flow cytometry for γ-carboxylated F9CH (and DsRed fluorescence). F9CH Gla and DsRed signals were calculated as percent of maximal, and corrected for background in naive HEK-Tet-ON parental cell line immunostained for γ-carboxylated F9CH.
Enantiomeric specificity of warfarin inhibition of F9CH γ-carboxylation
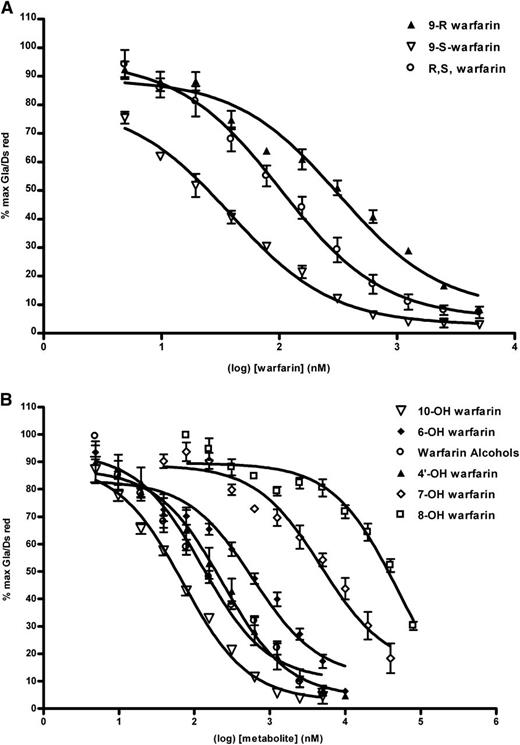
The anticoagulant potency of S-warfarin is typically reported to be threefold to fivefold greater than that of the R-warfarin enantiomer in vivo.22,23 Measuring the inhibitory effects of (S)-warfarin, racemic (R,S) warfarin, and (R)-warfarin separately on F9CH γ-glutamyl carboxylation in HEK 293-C3 cells (Figure 4A) enabled derivation of IC50 values of 25 nM, 125 nM, and 288 nM, respectively, which is in good agreement with their relative in vivo potencies as anticoagulants.
F9CH γ-carboxylation in HEK-C3 cells is differentially inhibited by warfarin enantiomers and metabolites. (A) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of S warfarin, R warfarin or R,S-warfarin were assayed by flow cytometry for γ-carboxylated F9CH and DsRed fluorescence as described. Log-transformed data from individual experiments (n = 4) were plotted. (B) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of racemic warfarin metabolites were assayed by flow cytometry for F9CH Gla and DsRed fluorescence as described. Curves represent log-transformed data from multiple experiments (n = 3-4).
F9CH γ-carboxylation in HEK-C3 cells is differentially inhibited by warfarin enantiomers and metabolites. (A) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of S warfarin, R warfarin or R,S-warfarin were assayed by flow cytometry for γ-carboxylated F9CH and DsRed fluorescence as described. Log-transformed data from individual experiments (n = 4) were plotted. (B) Doxycycline-induced, vitamin K1–supplemented HEK-C3 cells cultured in increasing concentrations of racemic warfarin metabolites were assayed by flow cytometry for F9CH Gla and DsRed fluorescence as described. Curves represent log-transformed data from multiple experiments (n = 3-4).
Inhibition of F9CH γ-carboxylation by phenolic and alcohol warfarin metabolites
Metabolism of warfarin in vivo results in the formation of the side-chain reduced alcohols and several monohydroxylated phenolic metabolites,1 a few of which have been examined in vivo in rodents to explore their anticoagulant activity. Figure 4B and Table 1 illustrate the structure-activity relationships for phenolic and alcohol metabolites and inhibition of vitamin K cycle activity. Only 10-hydroxywarfarin (IC50 = 83 nM) and the warfarin alcohols (IC50 = 132nM) approximate the potency of racemic warfarin in inhibiting F9CH γ-glutamyl carboxylation in HEK 293-C3 cells. The inhibitory potency of the other warfarin metabolites decreased in the following order: 4′-hydroxy > 6-hydroxy > 5-hydroxy > 7-hydroxy > 8-hydroxy.
Relative in vivo potency of phenolic and alcohol metabolites of warfarin
Finally, to obtain an estimate of the relative in vivo anticoagulant activities attributable to the warfarin enantiomers and metabolites, we determined [I]/IC50 values (Table 1) by dividing [I], defined as the upper range of published measurements for circulating in vivo anticoagulant concentrations,15,24 by the IC50 value obtained for F-IX Gla inhibition in C3 cells. Several warfarin enantiomers are highly protein bound in human plasma,25 therefore, we measured fraction unbound in human plasma for all the warfarin metabolites and corrected the reported circulating concentrations to reflect concentrations of free drug in vivo by calculating [I]u = [I] × fuplasma (Table 2). These measurements demonstrate that, like the parent drug, all warfarin metabolites are highly protein bound in human plasma (0.4%-3.4% unbound). We also measured the fraction unbound for warfarin and each metabolite in RGM, and found warfarin and its metabolites were much less tightly bound in RGM; 18% to 47% unbound, enabling calculation of IC50u = IC50 × fumedia. Calculated [I]u/IC50u values for warfarin enantiomers (Table 2) predict that S-warfarin would be expected to be 5× to 6× more potent in vivo than R-warfarin, in good agreement with the experimental in vivo data in humans.22,23 Using the same approach, only the warfarin alcohols (15% of S-warfarin activity) and 10-hydroxywarfarin (5% of S-warfarin activity) are metabolites that could be expected to contribute to the anticoagulant effects of the drug in vivo (Table 2).
Discussion
Our purpose was to establish a simple, quantitative cell-based model system to serve as a platform for the investigation of molecular mechanisms of drug- and diet-induced interactions affecting vitamin K–dependent blood-clotting processes. A recent success in this area is the development of a warfarin-inhibitable, AV12 cell system expressing a secreted chimeric protein C containing the Gla domain of factor IX26 with enzyme-linked immunosorbent assay quantitation. Similarly, activity of secreted recombinant factor IX was used to examine the relative warfarin sensitivities of several VKORC1 mutants overexpressed in HEK 293 cells.27 We have used a similar strategy here with an HEK 293–derived cell line. Key features of this approach include a bidirectional promoter enabling doxycycline-induced coexpression of cytoplasmic DsRed (as an internal standard) and the chimeric F9CH construct displaying the factor IX Gla domain on the cell surface. The commercial availability of an antibody specific for the Gla vs Glu conformation of factor IX thereby allows immunodetection and quantitation by flow cytometry.
Central to our goal was development of an experimental system that provides for extrapolation to the clinical situation in vivo. This has proven difficult for studies involving warfarin’s inhibition of VKOR, due to the complex interactions that exist among multiple vitamin K cycle components. For example, while S-warfarin is about 5× more potent an oral anticoagulant than R-warfarin, this difference in potency is not observed for inhibition of VKOR activity in vitro in rat liver microsomes.28 This discrepancy may reflect the use of a chemical reductant, DTT, to drive microsomal VKOR reduction while, in vivo, reduction of VKOR has been shown to be linked to oxidative protein folding.29,30 Notably, it has been shown that high concentrations of DTT release radiolabeled R-warfarin, relative to S-warfarin, from liver microsomes prepared from rats treated with racemic C14-warfarin.31 In the same study, differences in warfarin enantiomer potency emerged when microsomal VKOR reactions were driven by very high (42 mM) concentrations of DTT. These data suggest that differential liberation of warfarin enantiomers from VKOR by a more efficient in vivo reductant is the basis for the stereoselective differences in VKOR inhibition. If so, a stringent test of any model cell system would be to recapitulate the stereoselective differences in warfarin enantiomer potency for inhibition of clotting factor formation, assuming the endogenous reductant is expressed at significant levels in HEK cells. The data obtained here do, in fact, demonstrate a large (∼10-fold) difference in the IC50 for γ-glutamyl carboxylation in engineered HEK cells for S-warfarin compared with R-warfarin, suggesting that data obtained for other potential vitamin K antagonists using this system will be reflective of their potency in vivo.
Further underscoring the importance of relying on endogenous cellular components in measuring vitamin K cycle activity and warfarin inhibition, Fregin et al recently assessed the relative warfarin sensitivities of wild-type VKORC1 and clinically relevant mutants in HEK 293 cells.27 Their measurements of secreted active factor IX produced by the gene variants demonstrated relative VKOR activities that correlated well with in vivo anticoagulant dosing requirements for patients carrying the VKORC1-resistance alleles. In contrast, in a previous DTT-driven microsomal study of the same VKORC1 variants, VKOR activities did not correlate with their in vivo sensitivities to warfarin.11,32
With a validated cell model in hand, we examined the contribution of warfarin metabolites as potential contributors to vitamin K antagonism in vivo. Warfarin is metabolized to numerous phenolic metabolites by the P450 enzymes (CYP2C9, CYP3A4, CYP2C19, and CYP1A2) and to side-chain alcohols by carbonyl reductase.1 If any of these warfarin metabolites are potent vitamin K antagonists, drug-drug or drug-gene interactions involving the enzymes that generate them, or metabolize them further (eg, by glucuronidation), could affect a patient’s response to warfarin.33 Beyond the possible implications for warfarin patient response these structure-function studies may also provide insight into the molecular interactions between warfarin and VKOR that are required for enzyme inhibition.
In animal testing of warfarin metabolites conducted by Link and colleagues, administration of single doses of 6-, 7-, and 8-hydroxywarfarin to rats had negligible anticoagulant activity, whereas 4′-hydroxywarfarin elicited about 25% of the activity of the parent drug.34 These observations agreed with reports that structural modifications at C-3 were well tolerated, whereas changes to the phenyl ring of the 4-hydroxycoumarin nucleus abrogated oral anticoagulant activity.35 In agreement with these findings, halogenation of the 4-hydroxycoumarin phenyl ring was found to reduce anticoagulation potency in rabbits,36 suggesting that structural modifications to the 4-hydroxycoumarin nucleus of the molecule hinder interaction with the target enzyme, VKOR.
Our data provide additional refinement and extension of these structure-function relationships. In agreement with the early in vivo studies, 4′-hydroxywarfarin retained VKOR inhibitory activity and was only about threefold less potent than the parent drug. Also provided here are the first data regarding the pharmacological activity of 10-hydroxywarfarin, which is a VKOR inhibitor that is equipotent with warfarin itself. In contrast, hydroxylation on the phenyl ring of the 4-hydroxycoumarin reduced pharmacologic activity. 7-Hydroxywarfarin, the major in vivo human metabolite, was >100-fold less potent an inhibitor than warfarin. In contrast, the other major human phenolic metabolite, 6-hydroxywarfarin, was only about sixfold less potent as an inhibitor compared with parent drug. These latter data agree with regard to the relative potency described recently37 using rat liver microsomal VKOR activity as the reporter for chemical inhibition. This congruency of inhibitor potency data suggests that it is very likely that changes in γ-glutamyl carboxylation of the chimeric factor IX reporter in engineered HEK 293-C3 cells by warfarin and its major phenolic metabolites reflect their relative abilities to inhibit VKOR. Similar to 7-hydroxywarfarin, 8-hydroxywarfarin was ∼100-fold less potent an inhibitor than warfarin, whereas 5-hydroxywarfarin (not a known in vivo metabolite) was ∼20-fold less potent. Based on these considerations, we speculate that steric constraints for binding of warfarin to VKOR are greatest around the C-7 and C-8 positions, but much more flexibility exists in the region of the VKOR active site that contacts the C-6 position. The lower inhibitory potency of 5-hydroxywarfarin relative to 6-hydroxy warfarin may reflect increased steric hindrance, although electronic factors that decrease this metabolite’s propensity to form an anion at C-437 may also play a role because of intramolecular hydrogen bonding between hydroxyl groups at C-4 and C-5. This is evident in the crystal structure of 5-hydroxywarfarin.38
While the above analysis illuminates the nature of intrinsic inhibitory interactions between warfarin congeners and VKOR, prediction of the likely inhibitory effects in vivo requires consideration of metabolite IC50 values that have been corrected for steady-state plasma metabolite concentrations and differences in the degree of protein binding in plasma and cell media. Surprisingly, quantitation of all of warfarin’s circulating metabolites from anticoagulated patients has only been achieved recently using new chiral liquid chromatography-tandem mass spectrometry assays.15,24 We combined the data from these 2 chromatographic studies with the inhibitory potencies measured here in C3 cells to estimate [[I]/C50] values for warfarin and all of its monohydroxy metabolites (Table 1) and corrected this term further for protein binding. Compared with our fu measurements for 9S- and 9R-warfarin (0.4%), we found that most warfarin metabolites were less tightly bound in human plasma (2.1%-3.4% for 4′-OH, 6-OH, 7-OH, and 8-OH warfarin), in agreement with previous reports.39 The fu values we measured in human plasma for 5-OH and 10-OH warfarin and the warfarin alcohols were all <1.0%. In contrast, the fu in DMEM/10% FBS for all warfarin-related compounds tested was significantly higher than in human plasma. Therefore, when we adjust the [I]/IC50 values for maximal in vivo metabolite concentrations and fu in plasma and in culture medium, S-warfarin is predicted to be fivefold to sixfold more potent that R-warfarin as an anticoagulant, in agreement with in vivo observations, and the only metabolites predicted to contribute >4% of the pharmacologic activity of S-warfarin are the warfarin alcohols and 10-hydroxywarfarin.
Two caveats should be mentioned regarding the above extrapolations. First, because both of these alcohol metabolites contain 2 chiral centers, each metabolite is a mixture of 4 stereoisomers that could exhibit different potencies as vitamin K antagonists and/or variable plasma concentrations. Second, maximal circulating concentrations of the 10-hydroxywarfarin metabolite(s) are expected to be higher after treatment with CYP3A inducers,40 and so prediction of the contribution of 10-hydroxywarfarin to the in vivo effects of the parent drugs may be underestimated by the analysis presented here. Additional studies with individual warfarin metabolite stereoisomers will be necessary to resolve these points.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Institutes of General Medical Sciences grants P01GM32165 (A.E.R.), R01GM109743 (M.G.M. and A.E.R.), and U01GM92676 (J.A.H. and J.D.K.).
Authorship
Contribution: J.A.H., M.G.M., and J.D.K. designed and performed research, analyzed data, and wrote the paper; and A.E.R. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allan E. Rettie, Department of Medicinal Chemistry, School of Pharmacy, University of Washington, Box 357610, 1959 NE Pacific St, Seattle, WA 98195-7610; e-mail: rettie@uw.edu.