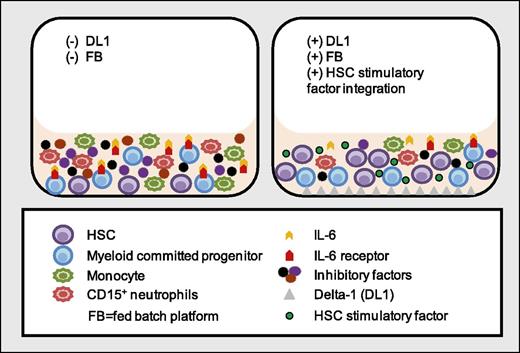
In this issue of Blood, Csaszar et al show that in ex vivo cultures of CD34+ cells, simultaneous reduction of interleukin-6 receptor (IL-6R) expression on myeloid progenitor cells by Delta-1 treatment combined with removal of soluble IL-6R using a dynamically fed culture system, reduces mature myeloid cell production, leading to enhanced expansion of primitive hematopoietic stem cells.1
Treatment of cultured CD34+ cells with Delta-1 in a fed-batch platform inhibits IL-6 cis- and trans-signaling in myeloid committed progenitor cells and reduces the production of mature inflammatory myeloid cells, which leads to depletion of inhibitory factors produced by these cells. Concomitant inhibition of mature myeloid cell production and depletion of inhibitory signals result in extensive ex vivo expansion of HSC from cultured CD34+ cells.
Treatment of cultured CD34+ cells with Delta-1 in a fed-batch platform inhibits IL-6 cis- and trans-signaling in myeloid committed progenitor cells and reduces the production of mature inflammatory myeloid cells, which leads to depletion of inhibitory factors produced by these cells. Concomitant inhibition of mature myeloid cell production and depletion of inhibitory signals result in extensive ex vivo expansion of HSC from cultured CD34+ cells.
Adult hematopoietic stem cells (HSCs) are among the best-characterized stem cells. In additional, more than 50 000 blood stem cell transplants are performed each year worldwide.2 Despite these successes, hematopoietic stem cell transplants (HSTs) are performed on only a fraction of patients who could potentially benefit from this life-saving therapy. This is particularly true of allogeneic HSTs, in which a suitably matched donor can be found for only 50% of candidates. Banked cord blood (CB) units are a potential source for HSCs, and the ever-increasing number of banked units can facilitate finding an HLA-matched graft. However, the low number of HSCs in CB has largely restricted CB HST to the pediatric setting. The identification of molecules that expand HSCs during ex vivo culture without losing their pluripotency is an important goal.
HSC fate is regulated by intrinsic signals from cytokines, growth factors, lipid molecules, and extrinsic cues that regulate self-renewal vs differentiation. Past strategies to expand HSC numbers ex vivo have focused primarily on using cytokines that directly target HSCs. These have led to variable degrees of HSC proliferation, but also to differentiation with resulting loss of HSC activity. Notch signaling has been shown to play a regulatory role in hematopoiesis, including rapid ex vivo HSC expansion and enhanced engraftment.3 However, until this study, it was unclear how Notch signaling enhanced HSC expansion. It was anticipated that intrinsic Notch signaling regulates HSC expansion and maintains pluripotency because Notch receptors are expressed on HSCs.4 In an elegant approach using highly purified, primitive HSC cultures (Lin−Rholow CD34+ 38−45−49f+; ∼20% HSCs) vs CD34+ cell cultures in which only 0.1% of cells is repopulating HSCs, the authors showed that Notch signaling indirectly regulates HSC expansion by minimizing the inhibitory impact of the ex vivo culture environment.1 That Delta-1 treatment enhances primitive cell (CD34+ 90+) expansion exclusively from heterogeneous CD34+ cell cultures but not cultures of highly purified HSCs suggests that the primitive HSCs are not the primary actor of Notch signaling. Notably, the production of CD14+ and CD15+ mature myeloid cells is reduced in Delta-1–treated CD34+ cultures.
Mechanistically, the authors demonstrate that Delta-1 reduces myeloid cell production from ex vivo–cultured CD34+ cells by reducing expression of IL-6R on myeloid-restricted progenitors. This prevents accumulation of monocytes and granulocytes and provides a more supportive environment for HSC expansion and self-renewal. However, in the absence of membrane IL-6R, myeloid cells can respond to IL-6 via trans-signaling in the presence of soluble IL-6R. Therefore, the authors used a fed-batch culture system that allows continuous depletion of soluble IL-6R from the culture, decoupling IL-6 trans-signaling.
The reduction of mature myeloid cells in the presence of Delta-1 is intriguing because CD14+ myeloid cells can exert inhibitory effects on ex vivo progenitor cell expansion.5 Together with a previous report showing the positive impact of myeloid cells on HSC in vivo, in particular monocytes/macrophages that preserve primitive HSCs,6 the current findings suggest differential impact of monocyte subsets on HSC function. CD14+ monocytes show functional heterogeneity and adopt inflammatory or anti-inflammatory phenotypes in response to environmental signals.7 Indeed, an increase in inflammatory monocyte/macrophage numbers is associated with impaired HSC function8 and expression of several inflammatory factors (eg, macrophage-inflammatory protein-1α, macrophage-inflammatory protein-1β, and IL-10 are upregulated during ex vivo HSC expansion).9 It is possible that cytokine cocktails in ex vivo culture medium (eg, stem cell factor, FMS-like tyrosine kinase-3 ligand, thrombopoietin) support generation of inflammatory myeloid cells, and inhibitory factors secreted by these cells impair HSC self-renewal–promoting differentiation. Delta-1 appears to enhance HSC recovery by suppressing inflammatory monocyte production. Because different subsets of monocytes can differentially affect HSC function, it will be important to characterize the ex vivo–generated CD14+ populations in Delta-1 cultures to perhaps further enhance primitive HSC recovery.
Ex vivo expansion of HSC has a significant clinical implication for stem cell therapies for life-threatening diseases. The studies by Csaszar et al highlight the importance of IL-6 signaling inhibition in primitive HSC ex vivo expansion and draw attention to the need to understand the complexities of present/future ex vivo culture systems with respect to the impact of non–stem cells on stem cells and to the fluctuation of stimulatory and inhibitory factors secreted by the endogenous culture environment. Selective removal of inhibitory cells/soluble factors from ex vivo–cultured CD34+ populations is a promising strategy to expand primitive HSCs for cell-based therapies.
Conflict-of-interest disclosure: L.M.P. has received consulting fees from Fate Therapeutics. The remaining author declares no competing financial interests.