Key Points
Lyn’s overexpression mediates resistance to apoptosis by promoting phosphorylation and dimerization of procaspase 8 in B-CLL cells.
Abstract
Lyn, a member of the group of tyrosine kinases named the Src family kinases (SFKs), is overexpressed, associated with an aberrant multiprotein complex and constitutively active in B-cell chronic lymphocytic leukemia (B-CLL) cells, resulting in a high level of tyrosine phosphorylation and contributing to their resistance to apoptosis. By using biochemical and bioinformatics tools, we identified procaspase-8 (procasp8), the caspase-8 zymogen, as a cytosolic target for Lyn in B-CLL cells, the phosphorylation of which at Tyr380 promotes the formation of an inactive procasp8 homodimer. This complex remains segregated in the cytosol and appears to be crucial in mediating the antiapoptotic function of Lyn in this disease. The significance of the Lyn-procasp8 axis in impairing apoptosis in B-CLL cells was further confirmed by pharmacological and genetic inhibition of procasp8, which drastically reduced the apoptosis induced by the SFK inhibitors PP2 and dasatinib. Our data highlight that Lyn’s dysregulated expression, activity, and localization in B-CLLs support resistance to cell demise by inhibiting an early player of apoptotic signaling, and potentially broaden the perspectives of developing new strategies for the treatment of this disease.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common leukemia in the Western world, with a heterogeneous clinical course varying from the stable, long-lasting indolent form to rapidly progressive disease and death. B-CLL is characterized by the clonal expansion with accumulation of mature CD5+/CD23+ B lymphocytes in the blood, lymphoid tissues, and bone marrow.1-4 Within the already known plethora of factors that are associated with the onset, progression, and prognosis of this disease are included an abnormal expression of antiapoptotic molecules, structural and functional features of the B-cell receptor (BCR) such as somatic mutations of the immunoglobulin variable heavy chain (IgVH) genes, and deregulation of the BCR-dependent signaling pathways.5,6 On the basis of the mutational status of IgVH, B-CLL patients can be classified into 2 major groups with different outcomes: one with mutated IgVH genes (M-CLL) and a relatively stable disease course and one with unmutated IgVH configuration (U-CLL) and a more aggressive clinical behavior.5-7
As to the abnormal functional state of the BCR, several lines of evidence indicate that the kinases participating in the “signalosomes,” the multi-protein complex associated with the elements at the cytosolic side of the BCR (eg, Lyn,8 Syk,9 and Btk10 ) and those propagating signaling (PI3K,11 PKC,12 and mitogen-activated protein kinase p3813 ) are hyperactive in B-CLL cells, which is also consistent with the view of a ligand-independent tonic BCR activation.14 Although most of these kinases did not exhibit apparent changes in expression, structure, or location, Lyn, a tyrosine kinase belonging to the Src family (Src family kinases [SFKs]), represents an exception, because it is overexpressed and segregated into 2 pools with different subcellular localization, one bound to the plasma membrane and acting as a molecular switch within the signalosome and the other as an integral component of an aberrant cytosolic complex.8,15,16 Within this complex, Lyn is associated with HSP90, this interaction being crucial for the altered turnover and the constitutive activity of Lyn itself, which in turn sustains the viability of leukemia cells by antagonizing apoptosis. In fact, agents capable of disrupting the complex, such as HSP90 inhibitors (geldanamycin [GA] and derivatives),15 are able to trigger apoptosis similar to SFK inhibitors (PP2, SU6656, and dasatinib),8,17,18 underscoring the function of this multi-protein assembly as a factor stabilizing Lyn in such a hyperactive form.
The aim of this work was to identify cytosolic proteins that act as antiapoptotic players in B-CLL cells after being phosphorylated by the constitutively active and delocalized pool of Lyn. Using biochemical methods and a bioinformatics approach, we established that in B-CLL cells, Lyn phosphorylates procaspase-8 (procasp8), the caspase-8 (casp8) zymogen, inducing the formation of a stable cytosolic inactive procasp8 dimer. Moreover, we found that procasp8 was activated by SFK inhibitors, with subsequent activation of the downstream caspase-dependent pathways, including both the extrinsic and the intrinsic apoptotic cascades, thus highlighting the role of procasp8 in mediating the antiapoptotic function of Lyn.
Materials and methods
Information concerning reagents, patient recruitment, cell separation and culture conditions subcellular fractionation, λ-PPase treatments, cell transfection, purification of procasp8, procasp8 phosphorylation assay, casp8 activity assay, apoptosis assays, bioinformatic analysis, 2-D gel electrophoresis, western blotting, and statistical analysis is detailed in the supplemental Data available on the Blood Web site. Written informed consent was obtained from all patients prior to sample collection according to the Declaration of Helsinki. The ethical approval for our study was obtained from the local ethical committee of Regione Veneto on chronic lymphocytic leukemia.
Results
Tyrosine phosphorylation of the cytosolic subproteome in B-CLL cells
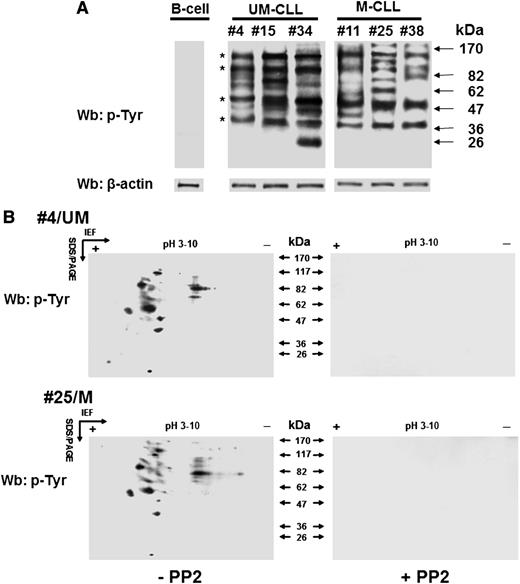
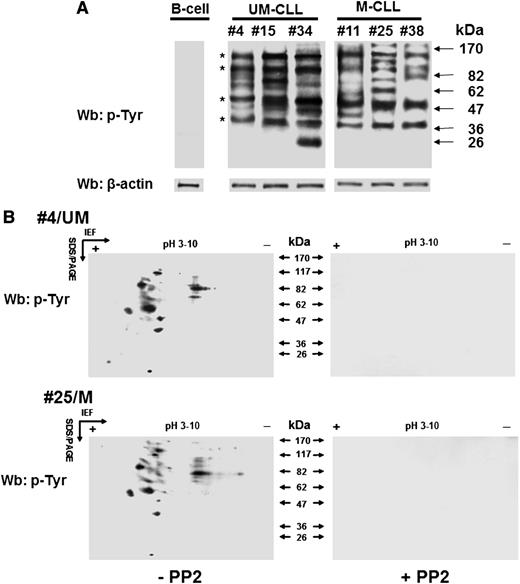
We previously showed that B-CLL cells exhibit a high level of tyrosine phosphorylation in the cytosol due to a constitutively active form of the SFK Lyn bound to an aberrant cytosolic complex, thereby contributing to the maintenance of the cancer phenotype; the other members of this family of tyrosine kinase remained quiescent.8,15,16 These findings led us to analyze cytosolic phosphotyrosine (pTyr) subproteomes from patients of the different prognostic subgroups (M-CLL and U-CLL) to detect possible molecular players related to the antiapoptotic function exerted by Lyn. Wb analysis with anti-pTyr antibody of the cytosol purified from freshly isolated B-CLL cells revealed a marked heterogeneity in pTyr patterns irrespective of the IgVH mutational status, a few bands being observed as a recurrent finding at an apparent molecular weight of 110, 90, 55, and 40 kDa in all samples. To characterize the potential proteins of interest, the cytosol of leukemia cells from 24 B-CLL patients, 12 U-CLL and 12 M-CLL was incubated in the absence or presence of the SFK inhibitor PP2 (10 µM) for 30 minutes and subject to 2D-polyacrylamide gel electrophoresis (2D-PAGE) using a linear wide range (pH 3-10) immobilized pH gradient in the first dimension for subsequent Wb analysis with anti-pTyr antibody. As shown in Figure 1B, most of the spots revealed with the anti-pTyr antibody disappeared after treatment with PP2 (left vs right panel), suggesting that tyrosine phosphorylation in the cytosol of B-CLL cells was dependent on Lyn. Overlapping results were also observed when the samples were treated with SU6656 (7.5 µM), a more selective SFK inhibitor (data not shown). Interestingly, the phosphoproteome was mostly distributed within a very narrow isoelectric point range, namely 4.5 to 5.5, with very few exceptions. A number of spots turned out to be common to all the samples (2 representative samples are shown), with molecular weights compatible with the protein bands previously detected in Figure 1A.
Lyn aberrant activity generates varying tyrosine phosphorylation patterns of the cytosolic subproteome in B-CLL cells. (A) Cytosol of freshly isolated B cells from normal donors and B-CLL patients distributed between the UM-CLL (patients 4, 15, and 34) and M-CLL (patients 11, 25, and 38) subsets was analyzed by Wb analysis with anti-pTyr antibody and probed with anti-β-actin antibody as a loading control. Asterisks indicate recurrent bands within the B-CLL samples. (B) Cytosol of freshly isolated B-CLL cells, purified from patients in different clinical stages and belonging to the unmutated (UM-CLL) and mutated (M-CLL) IgVH subsets (patients 4 and 25, respectively, in this figure) and cultured in the absence (left panels) or presence (right panels) of 10 μM PP2, was separated by 2D-PAGE using a linear wide range (pH 3-10) immobilized pH gradient in the first dimension for subsequent Wb analysis with anti-pTyr antibody. The figure is representative of samples from 6 normal donors and 24 CLL patients belonging to UM-CLL and M-CLL subsets, each of which comprised 12 samples. IEF, isoelectric focusing; SDS/PAGE, sodium dodecyl sulfate/polyacrylamide gel electrophoresis.
Lyn aberrant activity generates varying tyrosine phosphorylation patterns of the cytosolic subproteome in B-CLL cells. (A) Cytosol of freshly isolated B cells from normal donors and B-CLL patients distributed between the UM-CLL (patients 4, 15, and 34) and M-CLL (patients 11, 25, and 38) subsets was analyzed by Wb analysis with anti-pTyr antibody and probed with anti-β-actin antibody as a loading control. Asterisks indicate recurrent bands within the B-CLL samples. (B) Cytosol of freshly isolated B-CLL cells, purified from patients in different clinical stages and belonging to the unmutated (UM-CLL) and mutated (M-CLL) IgVH subsets (patients 4 and 25, respectively, in this figure) and cultured in the absence (left panels) or presence (right panels) of 10 μM PP2, was separated by 2D-PAGE using a linear wide range (pH 3-10) immobilized pH gradient in the first dimension for subsequent Wb analysis with anti-pTyr antibody. The figure is representative of samples from 6 normal donors and 24 CLL patients belonging to UM-CLL and M-CLL subsets, each of which comprised 12 samples. IEF, isoelectric focusing; SDS/PAGE, sodium dodecyl sulfate/polyacrylamide gel electrophoresis.
Search for potential cytosolic Lyn’s substrates combining 2D-PAGE and bioinformatic analysis
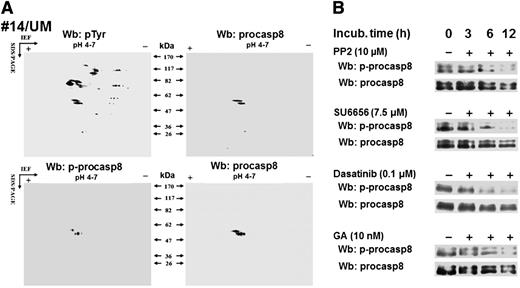
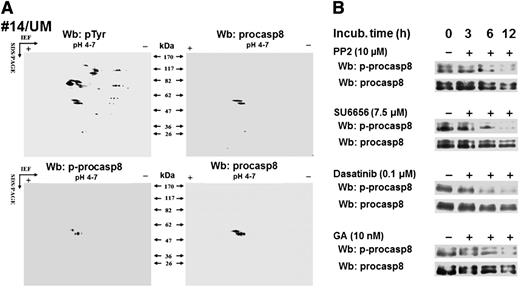
To identify Lyn’s cytosolic substrates potentially involved in the antiapoptotic mechanisms in B-CLLs, we adopted a bioinformatics approach, as described in the supplemental Data by applying the characteristics of the 2-dimensional phosphoproteome (isoelectric point, molecular weight, and subcellular location) as selective criteria, with the final output consisting of 7 tyrosine phosphorylated proteins (see the table included as an inset in supplemental Figure 1).19-27 Among these, casp8, a cysteine protease known to trigger the extrinsic apoptotic pathway, is described as inhibited when the zymogen form, procasp8, is phosphorylated by SFKs at Tyr380.21,22 To verify whether procasp8 was included in the set of Lyn-dependent tyrosine phosphorylated cytosolic substrates in B-CLL, the cytosol of leukemia cells from 12 patients (6 U-CLL and 6 M-CLL) underwent 2D-PAGE using a narrow linear range (pH 4-7) immobilized pH gradient in the first dimension to improve the resolution of the pTyr spots followed by Wb analysis with anti-pTyr antibody. Figure 2A shows that the doublet detected by the anti-procasp8 antibody (right panels) corresponded to those on the blots revealed with anti-pTyr (upper left panel) and anti-phospho-procasp8 (p-procasp8) antibodies (lower left panel), indicating that p-procasp8 is a component of the pTyr subproteome and is phosphorylated at Tyr380, the residue targeted by SFKs. To assess the role of Lyn in the phosphorylation of this tyrosine residue, we treated B-CLL cells with PP2 and SU6656 over time and eventually observed a significant decrease in the level of p-procasp8 (Figure 2B). Similar results were achieved by using dasatinib, an orally administered small molecule inhibitor of multiple tyrosine kinases, including SFKs, which is indicated for the treatment of chronic myeloid leukemia and acute lymphoblastic leukemia.17
Procasp8 emerges as Lyn’s substrate within B-CLL cytosolic tyrosine phosphoproteome. (A) Cytosol purified from freshly isolated B cells from 12 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets was separated by 2D-PAGE using a linear narrow range (pH 4-7) immobilized pH gradient in the first dimension for subsequent Wb analysis with anti-pTyr and anti-p-procasp8 antibodies, respectively. Blots were then stripped and reprobed with anti-procasp8 antibody. The figure, though showing the Wb patterns of patient 14, is representative of all the samples tested. (B) Whole B-cell lysates of freshly isolated B-CLL cells cultured in the absence or presence of 10 μM PP2, 7.5 μM SU6656, 0.1 μM dasatinib, or 10 nM GA at different time points underwent Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies. The figures are representative of samples from 10 CLL patients belonging to 2 different subsets UM and M-CLL composed of 5 samples each.
Procasp8 emerges as Lyn’s substrate within B-CLL cytosolic tyrosine phosphoproteome. (A) Cytosol purified from freshly isolated B cells from 12 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets was separated by 2D-PAGE using a linear narrow range (pH 4-7) immobilized pH gradient in the first dimension for subsequent Wb analysis with anti-pTyr and anti-p-procasp8 antibodies, respectively. Blots were then stripped and reprobed with anti-procasp8 antibody. The figure, though showing the Wb patterns of patient 14, is representative of all the samples tested. (B) Whole B-cell lysates of freshly isolated B-CLL cells cultured in the absence or presence of 10 μM PP2, 7.5 μM SU6656, 0.1 μM dasatinib, or 10 nM GA at different time points underwent Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies. The figures are representative of samples from 10 CLL patients belonging to 2 different subsets UM and M-CLL composed of 5 samples each.
Moreover, to establish whether the subpopulation of Lyn associated with the aberrant cytosolic multi-protein complex was responsible for procasp8 phosphorylation, we treated B-CLL cells with GA, an inhibitor of the protein chaperone Hsp90 that directly binds to Lyn and stabilizes it into the constitutively active form within the complex itself. As previously observed, this agent caused the disruption of the complex, hence inactivating the cytosolic pool of Lyn and leaving the microsomal one unaffected.15 The use of GA at different time points proved as effective as PP2, SU6656, and dasatinib in abolishing procasp8 phosphorylation, confirming the role of the anomalous activity of Lyn in the cytosol of B-CLL cells (Figure 2B).
Procasp8 is equally expressed and functionally inhibited in U- and M-CLL cells
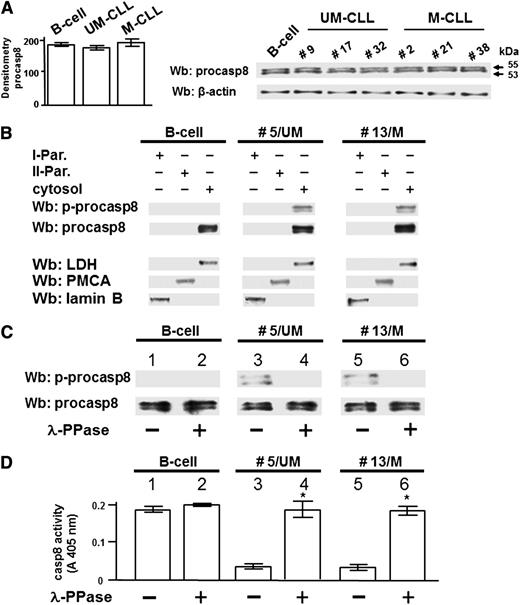
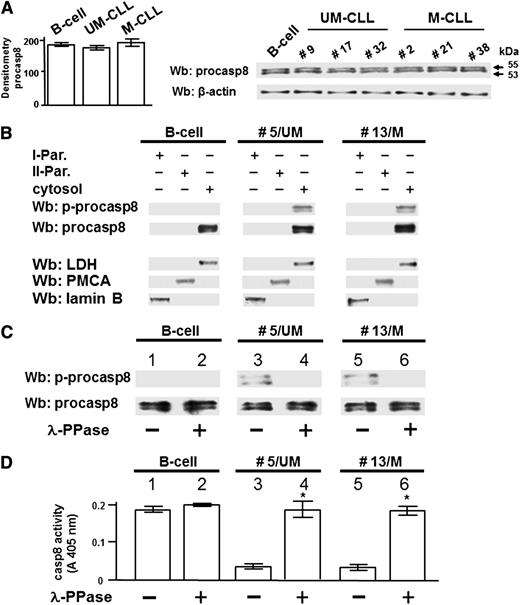
The extrinsic apoptotic pathway is generally initiated upon recruitment of the procasp8 to the Death-Inducing Signaling Complex (DISC) in response to ligation of death receptors, with subsequent dimerization, proteolytic cleavage, and full activation of casp8.28-30 Accumulating evidence has highlighted an emerging role of casp8 as regulator of other cellular processes with nonapoptotic functions.31-33 To better define how phosphorylation by Lyn could affect the function of procasp8 in B-CLL, we assessed its expression in the leukemia cells of all the 40 patients recruited in this work compared with 6 healthy donors. All the total cell lysates exhibited an equal protein level of procasp8 in agreement with the evidence that this protease seldom displays decreased expression in tumors (Figure 3A).34 We then analyzed the subcellular localization of procasp8 in normal B lymphocytes and B-CLL cells by cellular fractionation after differential centrifugation, with cytosol and microsomes (II particulate) being well separated from the other subcellular fractions (I particulate). Procasp8 was exclusively localized to the cytosol of both normal B lymphocytes and B-CLL cells independently of the clinical stage and the mutational status of IgVH (Figure 3B). Besides these similarities, the distinguishing feature of B-CLL cells was the phosphorylation of the protease at Tyr380, which was due to the action of Lyn as component of the aberrant cytosolic complex. To verify whether phosphorylation inhibited the proteolytic activity of casp8, as elsewhere reported,15 the cytosol of B lymphocytes from 3 healthy donors and 20 B-CLL patients (10 U-CLL and 10 M-CLL) incubated in the absence or presence of λ-PPase, underwent Wb analysis with anti-pTyr380 and anti-procasp8 antibodies and was tested for caspase activity. The phosphorylation state of Tyr380 appeared to be crucially implicated in caspase activity, the protease being inhibited when phosphorylated both in U- and M-CLL cells (Figure 3C middle and right panels; Figure 3D middle and right histograms), which did not occur in normal B cells (Figure 3C left panel; Figure 3D left histogram). Furthermore, this negative effect proved to be reversible, as demonstrated by the use of λ-PPase, which resulted in the dephosphorylation and subsequent activation of procasp8.
Lyn-dependent tyrosine phosphorylation inhibits casp8 activity in B-CLL cells. (A) Whole B-cell lysates from 6 normal donors and all B-CLL patients distributed between the UM-CLL and M-CLL subsets (supplemental Table 1) were analyzed by Wb analysis with anti-procasp8 antibody, and the blots were reprobed with anti-β-actin antibody as a loading control. Pooled densitometric analysis (arbitrary units) of the Wb bands of the immunoblots are shown (left panel). Wb analysis representative of one normal donor and B-CLL patients equally distributed between the UM-CLL (patients 9, 17, and 32) and M-CLL subsets (patients 2, 21, and 38) are shown in the right panel. (B) Equivalent amounts of protein of the different subcellular fractions (first particulate fraction, I-Par., corresponding to nuclei and mitochondria; second particulate fraction, II-Par., corresponding to microsomes; and cytosol) from normal donors and B-CLL patients belonging to the UM-CLL and M-CLL subsets (represented by patients 5 and 13, respectively, compared with one normal donor in this figure) were analyzed by Wb analysis with anti-procasp8 antibody, and the blots were reprobed with antibodies against lactate dehydrogenase (LDH, cytosolic marker), plasma membrane Ca2+ ATPase (PMCA, plasma membrane marker), and lamin (nuclear marker) as loading controls. The figure is representative of samples from 3 normal donors and 10 CLL patients belonging to 2 different subsets UM-CLL and M-CLL composed of 5 samples each. (C-D) Cytosol from normal donors and B-CLL patients belonging to the UM-CLL and M-CLL subsets (patients 5 and 13, respectively, compared with one normal donor in this figure) were incubated in the absence (lanes 1, 3, and 5) or presence of λ-PPase (lanes 2, 4, and 6) for 1 hour at 37°C. Equivalent amounts of protein underwent Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies (C) or assayed for in vitro casp8 activity (D). The figure is representative of samples from 3 normal donors and 20 B-CLL patients belonging to the UM-CLL and M-CLL subsets composed of 10 samples each (*P < .05, lanes 3 vs 4 and 5 vs 6).
Lyn-dependent tyrosine phosphorylation inhibits casp8 activity in B-CLL cells. (A) Whole B-cell lysates from 6 normal donors and all B-CLL patients distributed between the UM-CLL and M-CLL subsets (supplemental Table 1) were analyzed by Wb analysis with anti-procasp8 antibody, and the blots were reprobed with anti-β-actin antibody as a loading control. Pooled densitometric analysis (arbitrary units) of the Wb bands of the immunoblots are shown (left panel). Wb analysis representative of one normal donor and B-CLL patients equally distributed between the UM-CLL (patients 9, 17, and 32) and M-CLL subsets (patients 2, 21, and 38) are shown in the right panel. (B) Equivalent amounts of protein of the different subcellular fractions (first particulate fraction, I-Par., corresponding to nuclei and mitochondria; second particulate fraction, II-Par., corresponding to microsomes; and cytosol) from normal donors and B-CLL patients belonging to the UM-CLL and M-CLL subsets (represented by patients 5 and 13, respectively, compared with one normal donor in this figure) were analyzed by Wb analysis with anti-procasp8 antibody, and the blots were reprobed with antibodies against lactate dehydrogenase (LDH, cytosolic marker), plasma membrane Ca2+ ATPase (PMCA, plasma membrane marker), and lamin (nuclear marker) as loading controls. The figure is representative of samples from 3 normal donors and 10 CLL patients belonging to 2 different subsets UM-CLL and M-CLL composed of 5 samples each. (C-D) Cytosol from normal donors and B-CLL patients belonging to the UM-CLL and M-CLL subsets (patients 5 and 13, respectively, compared with one normal donor in this figure) were incubated in the absence (lanes 1, 3, and 5) or presence of λ-PPase (lanes 2, 4, and 6) for 1 hour at 37°C. Equivalent amounts of protein underwent Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies (C) or assayed for in vitro casp8 activity (D). The figure is representative of samples from 3 normal donors and 20 B-CLL patients belonging to the UM-CLL and M-CLL subsets composed of 10 samples each (*P < .05, lanes 3 vs 4 and 5 vs 6).
Phosphorylation of Tyr380 as a trigger for the assembling of inactive procasp8 dimer in B-CLL
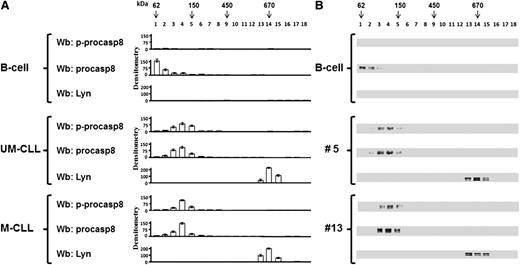
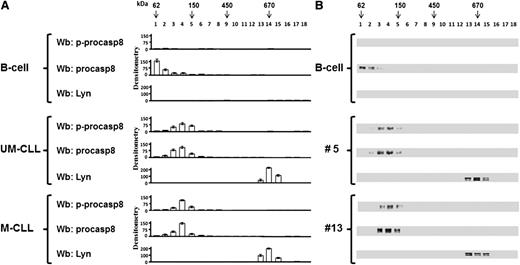
Because it is known that the mechanism of activation of casp8 results from the formation of a dimer with structural rearrangements of the active site,35-37 we wondered whether phosphorylation by Lyn might affect the dimerization of procasp8 in B-CLL cells, in addition to the activity thereof, as compared with normal B lymphocytes. To address this issue, the cytosol of B lymphocytes from 2 healthy donors and 14 B-CLL patients (7 U-CLL and 7 M-CLL) was fractionated on a glycerol linear gradient, and the resulting fractions were tested for p-procasp8, procasp8, and Lyn by Wb analysis. Procasp8 was mostly detected at molecular weights compatible with a monomeric form in normal B cells (Figure 4 upper panel), whereas the protease in B-CLL cells, irrespective of the IgVH mutational status (UM or M, patients no. 5 and 13, respectively), mainly exhibited a shift to molecular weights suggestive of a dimer (middle and lower panels). Within this complex, procasp8 turned out to be phosphorylated at Tyr380, in contrast with the nonphosphorylated and monomeric form in normal B lymphocytes, suggesting a correlation between the assembling and the phosphorylation state of procasp8. Importantly, the presence of protein partners described to interact with procasp8, such as the p85 subunit of phosphoinositide 3-kinase, which recognizes pTyr380 within the Y380LEM motif,38 and cellular FLICE-like inhibitory protein (cFLIP), which binds procasp8 activation through homophilic binding to the death effector domain (DED),39 was excluded by immunoprecipitation assays (data not shown). Moreover, although another member of the Src family, Src, has been demonstrated to interact with the procasp8 DED irrespective of the phosphorylation state of procasp8,34 we assessed that the protease did not interact with Lyn in the cytosol, which instead is part of an aberrant cytosolic 600-kDa complex (Figure 4), as previously described by us.15
Procasp8 presents as a dimer in B-CLL cells. Cytosol of 15 × 106 freshly isolated B cells from 2 normal donors and 14 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets underwent a linear glycerol gradient (10% to 40%) and was centrifuged 18 hours at 100 000g in an SW60Ti rotor (Beckman Coulter) at 4°C. (A) Eighteen fractions (200 μL each) of the glycerol gradient were collected from top and underwent Wb analysis with anti-p-procasp8, anti-procasp8, and anti-Lyn antibodies. Densitometric analysis (arbitrary units) of the Wb bands of the immunoblots from 2 normal donors and 14 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets are shown. (B) Wb analysis of one normal donor compared with that of UM-CLL (no. 5) and M-CLL (no. 13) subsets are shown.
Procasp8 presents as a dimer in B-CLL cells. Cytosol of 15 × 106 freshly isolated B cells from 2 normal donors and 14 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets underwent a linear glycerol gradient (10% to 40%) and was centrifuged 18 hours at 100 000g in an SW60Ti rotor (Beckman Coulter) at 4°C. (A) Eighteen fractions (200 μL each) of the glycerol gradient were collected from top and underwent Wb analysis with anti-p-procasp8, anti-procasp8, and anti-Lyn antibodies. Densitometric analysis (arbitrary units) of the Wb bands of the immunoblots from 2 normal donors and 14 B-CLL patients equally distributed between the UM-CLL and M-CLL subsets are shown. (B) Wb analysis of one normal donor compared with that of UM-CLL (no. 5) and M-CLL (no. 13) subsets are shown.
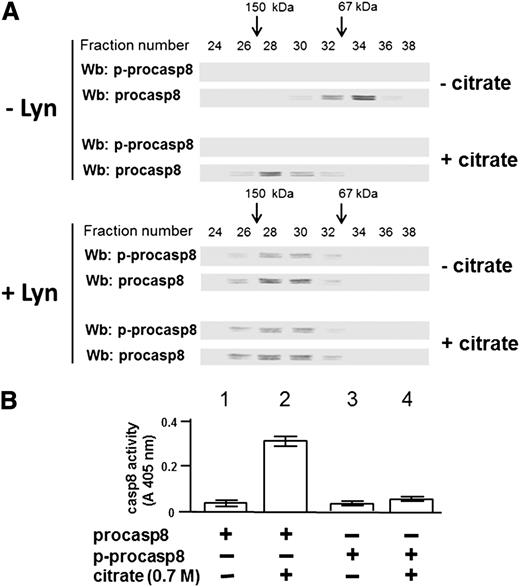
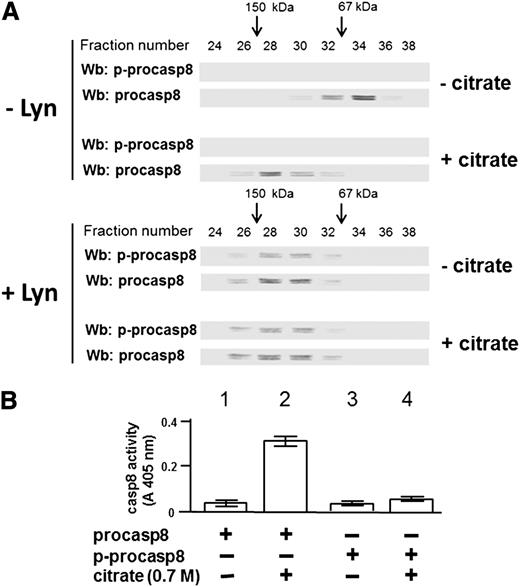
To better define how tyrosine phosphorylation Lyn could lead to procasp8 dimerization, procasp8 was phosphorylated de novo in the presence of Lyn after purification from the cytosol of 30 B-CLL patients performed in the absence of phosphatase inhibitors both in the lysis and the chromatography buffers, which resulted in dephosphorylation of the protease by the endogenous phosphatases (see the supplemental Data for further details). This experiment yielded a stoichiometry of ∼1 mol phosphate per mole of protein, corroborating the notion that procasp8 harbors only one Lyn-dependent phosphorylatable site, namely, Tyr380 (supplemental Figure 2). To explore how phosphorylation and/or dimerization could influence the proteolytic activity of procasp8, the purified protease underwent gel filtration as untreated after incubation in the presence of sodium citrate, a kosmotropic salt already shown to promote activation of caspases,40 and under the 2 above conditions after incubation with Lyn, so that its molecular weight could be estimated from the elution profile. Sodium citrate, as expected, provoked dimerization and activation of the unphosphorylated form of procasp8, as assessed from the elution profile shown in Figure 5A (compare the western blots in the upper panels, − and + citrate, −Lyn) and from the proteolytic activity graphed in Figure 5B. Regardless of the treatment with the kosmotrope, phosphorylation of procasp8 caused dimerization (compare the western blots in the lower panels, − and + citrate, + Lyn) but prevented activation (see far right bars in the bar graph), confirming a critical role of phosphorylation of Tyr380 in regulating the conformational status and function of procasp8.
Phosphorylation at Tyr380 by Lyn results in the formation of an inactive procasp8 dimer. (A) The elution profile of procasp8, as such or phosphorylated by Lyn and subsequently incubated in the absence or presence of 0.7 M sodium citrate, from a Superdex 200 HR column was determined by Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies. (B) Equivalent protein amounts of procasp8, as such or phosphorylated by Lyn and subsequently incubated in the absence or presence of 0.7 M sodium citrate, were assayed for in vitro casp8 activity as described in the supplemental Data.
Phosphorylation at Tyr380 by Lyn results in the formation of an inactive procasp8 dimer. (A) The elution profile of procasp8, as such or phosphorylated by Lyn and subsequently incubated in the absence or presence of 0.7 M sodium citrate, from a Superdex 200 HR column was determined by Wb analysis with anti-p-procasp8 and anti-procasp8 antibodies. (B) Equivalent protein amounts of procasp8, as such or phosphorylated by Lyn and subsequently incubated in the absence or presence of 0.7 M sodium citrate, were assayed for in vitro casp8 activity as described in the supplemental Data.
Procasp8 inhibition by Lyn-dependent phosphorylation prevents B-CLL cell apoptosis
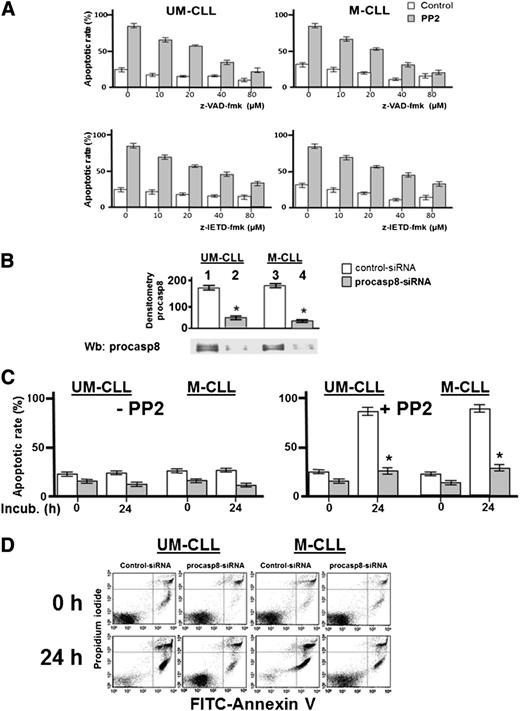
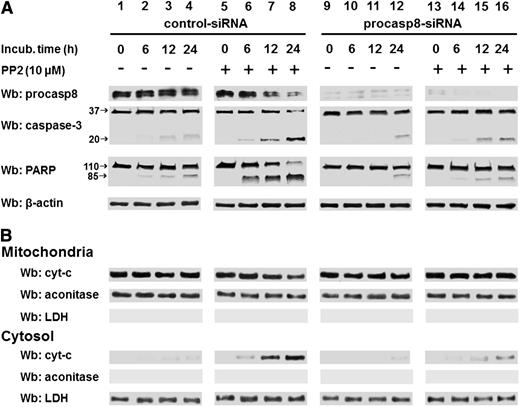
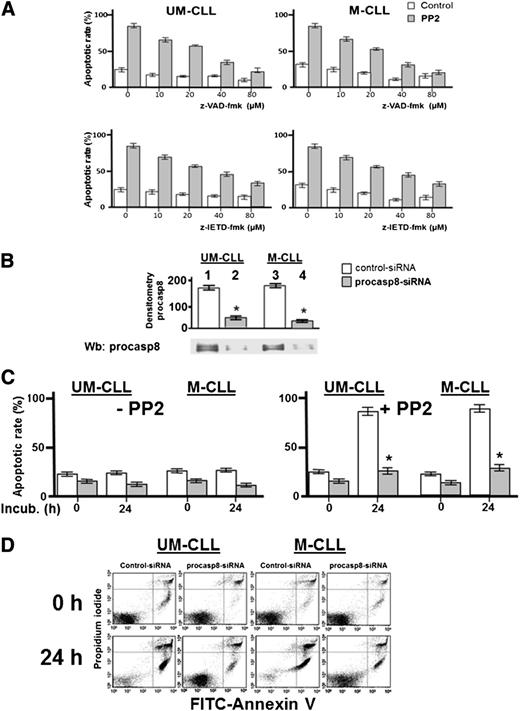
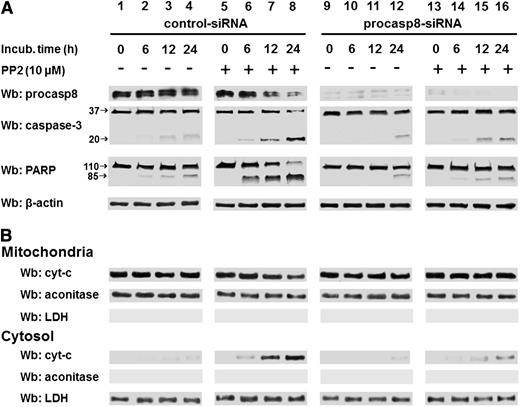
The phosphorylation-dependent impairment of the proteolytic activity of procasp8 raised the question of whether this is one of mechanisms by which Lyn counteracts apoptosis in B-CLL cells, as suggested by the ability of PP2 to trigger caspase-dependent apoptosis.8 This is a critical point, especially in view of the fact that the extrinsic apoptotic pathway, in which casp8 normally acts as initiator caspase, is defective in B-CLL due to the low expression and refractoriness of death receptors.41,42 To assess the contribution of casp8 in mediating the apoptotic process induced by SFK inhibitors, we incubated freshly isolated B-CLL cells from 16 B-CLL patients (8 U-CLL and 8 M-CLL) with increasing concentrations of either the pan-caspase inhibitor benzyloxycarbonyl-valyl-alanyl-aspartyl-[O-methyl]-fluoromethyl ketone (z-VAD-fmk) or the specific procasp8 inhibitor benzyloxycarbonyl-isoleucyl-glutamyl-[O-methyl]-threonyl-aspartyl-[O-methyl]-fluoromethyl ketone (z-IETD-fmk) in the absence or presence of 10 μM PP2 for 24 hours. As shown in the upper panel of Figure 6A, the cell death caused by PP2 was countered in a dose-dependent manner by z-VAD-fmk with a high degree of protection against apoptosis at concentrations of 40 to 80 μM. Furthermore, though not reaching the same protective effect of z-VAD-fmk, z-IETD-fmk was also able to efficiently counteract PP2-dependent apoptosis (Figure 6A lower panel). Although this result tempted us to ascribe the main role in PP2-induced apoptosis to casp8, we further explored how procasp8 could be involved in this process by knocking down this protease in B-CLL cells incubated in the absence and presence of PP2. This experimental procedure confirmed that the expression of procasp8 is a prerequisite for apoptosis to be unleashed by PP2 treatment in B-CLL cells, which would be prevented by the inhibitory effect of the phosphorylation by Lyn (Figure 6C-D). To evaluate how these molecular events could impinge on the apoptotic cascade and better define the role and location of procasp8 therein, we also analyzed the fate of a few known factors implicated in cell death. When treated with control small interfering RNAs (siRNAs) and PP2 (Figure 7A, lanes 5-8 vs 1-4), B-CLL cells exhibited cleavage of procasp8 and caspase-3, traditionally considered initiator and effector caspases, respectively, and of poly ADP ribose polymerase (PARP), the terminal substrate of caspase-3. On the other hand, procasp8 knockdown dramatically reduced the breakdown of caspase-3 and PARP even when PP2 was applied, indicating that procasp8 is functionally located upstream of the activation of caspase-3 and again confirming that Lyn exerts a negative control over procasp8 in B-CLL cells.
Pharmacologic and genetic inhibition of procasp8 reduces the apoptotic effect of Lyn inhibition. (A) Freshly isolated B-CLL cells were cultured in the absence or presence of 10 μM PP2 with increasing concentrations of z-VAD-fmk (upper panels) or z-IETD-fmk (lower panels) for 48 hours, and cell apoptosis was analyzed by annexin V–propidium iodide (PI) flow cytometry. Quadrant analysis after flow cytometry was expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 16 B-CLL patients belonging to 2 different UM-CLL and M-CLL subsets (left and right panels, respectively) composed of 8 samples each. Compared with the effect of PP2 alone, changes were significant starting at 10 μM of z-VAD-fmk or z-IETD-fmk, respectively. (B) Freshly isolated B-CLL cells were transfected by nucleofection with either the negative control (lanes 1 and 3) or procasp8-siRNAs (lanes 2 and 4) and cultured for 48 hours in complete medium. Cell lysates then underwent Wb analysis with anti-procasp8 antibody to assess the efficacy of siRNA technology. Expression of procasp8 in the whole cell of procasp8 siRNA transfected cells compared with that from siRNA control transfected cells was significantly decreased (*P < .05, lanes 1, 2, and 3 vs 4). (C) Freshly isolated B-CLL cells were transfected by nucleofection with either the negative control (white bar) or procasp8-siRNAs (shaded bar), cultured for 48 hours in complete medium, and subsequently incubated for 0 or 24 hours in the absence (left panel) or presence (right panel) of 10 µM PP2. Cell apoptosis was then analyzed by annexin V–PI flow cytometry, with quadrant analysis being expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 16 B-CLL patients belonging to 2 different UM-CLL and M-CLL subsets (left and right panels, respectively) composed of 8 samples each. Compared with the effect of PP2, changes due to procasp8 siRNA were statistically significant (*P < .05 or higher degree of significance). (D) Annexin V–PI flow cytometry analysis of 2 samples representative of the UM-CLL and M-CLL groups from the right panel of C. FITC, fluorescein isothiocyanate.
Pharmacologic and genetic inhibition of procasp8 reduces the apoptotic effect of Lyn inhibition. (A) Freshly isolated B-CLL cells were cultured in the absence or presence of 10 μM PP2 with increasing concentrations of z-VAD-fmk (upper panels) or z-IETD-fmk (lower panels) for 48 hours, and cell apoptosis was analyzed by annexin V–propidium iodide (PI) flow cytometry. Quadrant analysis after flow cytometry was expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 16 B-CLL patients belonging to 2 different UM-CLL and M-CLL subsets (left and right panels, respectively) composed of 8 samples each. Compared with the effect of PP2 alone, changes were significant starting at 10 μM of z-VAD-fmk or z-IETD-fmk, respectively. (B) Freshly isolated B-CLL cells were transfected by nucleofection with either the negative control (lanes 1 and 3) or procasp8-siRNAs (lanes 2 and 4) and cultured for 48 hours in complete medium. Cell lysates then underwent Wb analysis with anti-procasp8 antibody to assess the efficacy of siRNA technology. Expression of procasp8 in the whole cell of procasp8 siRNA transfected cells compared with that from siRNA control transfected cells was significantly decreased (*P < .05, lanes 1, 2, and 3 vs 4). (C) Freshly isolated B-CLL cells were transfected by nucleofection with either the negative control (white bar) or procasp8-siRNAs (shaded bar), cultured for 48 hours in complete medium, and subsequently incubated for 0 or 24 hours in the absence (left panel) or presence (right panel) of 10 µM PP2. Cell apoptosis was then analyzed by annexin V–PI flow cytometry, with quadrant analysis being expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 16 B-CLL patients belonging to 2 different UM-CLL and M-CLL subsets (left and right panels, respectively) composed of 8 samples each. Compared with the effect of PP2, changes due to procasp8 siRNA were statistically significant (*P < .05 or higher degree of significance). (D) Annexin V–PI flow cytometry analysis of 2 samples representative of the UM-CLL and M-CLL groups from the right panel of C. FITC, fluorescein isothiocyanate.
PP2-mediated apoptosis is countered by specific procasp-8 inhibition. (A) Freshly isolated B-CLL cells were transfected by nucleofection with either the control-siRNA (lanes 1-8) or procasp8-siRNAs (9-16), cultured for 48 hours in complete medium, and subsequently incubated in the absence (lanes 1-4 and 9-12) or presence (lanes 5-8 and 13-16) of 10 µM PP2 at different time points. After such treatment, cells were lysed and analyzed by Wb analysis with anti-procasp8, anti caspase-3, and anti-PARP antibodies as well as anti-β-actin antibody as a loading control. (B) Freshly isolated B-CLL cells were cultured as described in A. After treatment with the aforementioned inhibitor, cells underwent differential centrifugation to separate mitochondrial and cytosolic fractions. Equivalent protein amounts were assayed by Wb analysis with anti-cyt-c antibody as well as with anti-aconitase and anti-LDH antibodies to assess the purity of the fractions.
PP2-mediated apoptosis is countered by specific procasp-8 inhibition. (A) Freshly isolated B-CLL cells were transfected by nucleofection with either the control-siRNA (lanes 1-8) or procasp8-siRNAs (9-16), cultured for 48 hours in complete medium, and subsequently incubated in the absence (lanes 1-4 and 9-12) or presence (lanes 5-8 and 13-16) of 10 µM PP2 at different time points. After such treatment, cells were lysed and analyzed by Wb analysis with anti-procasp8, anti caspase-3, and anti-PARP antibodies as well as anti-β-actin antibody as a loading control. (B) Freshly isolated B-CLL cells were cultured as described in A. After treatment with the aforementioned inhibitor, cells underwent differential centrifugation to separate mitochondrial and cytosolic fractions. Equivalent protein amounts were assayed by Wb analysis with anti-cyt-c antibody as well as with anti-aconitase and anti-LDH antibodies to assess the purity of the fractions.
In addition, because casp8 is also known to elicit the intrinsic apoptotic pathway in response to the activation of death receptors by cleaving Bid, a BH3 domain-containing proapoptotic Bcl2 family member (which in turn mediates mitochondrial damage and release of cytochrome c [cyt-c] into the cytosol),30 we evaluated the content of cyt-c in the 2 compartments after treatment with PP2 upon silencing procasp8. As shown in Figure 7B, although PP2 treatment provoked a decrease in the level of the mitochondrial cyt-c with a concurrent increase of the cytosolic fraction (lanes 5-8 vs 1-4), procasp8 knockdown almost completely prevented cyt-c from being released from mitochondria, which is consistent with the notion of casp8 as a critical factor bridging the 2 arms of apoptosis. Notably, overlapping results were achieved by using dasatinib under the above-mentioned experimental conditions (supplemental Figure 3). In light of this data, we attempted to assess whether the casp8-Bid axis was required for the translocation of cyt-c to the cytosol upon PP2 treatment. Supplemental Figure 4 shows that the protein level of Bid in B-CLL cells was slightly lower than in normal B lymphocytes (supplemental Figure 4A) and that Bid knockdown in B-CLL cells did not prevent PP2-induced apoptosis (supplemental Figure 4B-C). Thus, PP2 treatment did not turn out to cause Bid truncation (date not shown), suggesting that the permeabilization of the mitochondrial outer membrane due to casp8 activation under these experimental conditions is mediated by an alternative mechanism and yet-to-be identified players. Importantly, procasp8 knockdown did not affect the integrity of the mitochondrial events of the intrinsic apoptotic pathway downstream of Bid truncation, as shown by the cell death induced by treatment with ABT-737, a BH3-only mimetic that binds with high affinity to and counters the anti-apoptotic function of Bcl-2 and Bcl-xL,43 again stressing the role of casp8 in taking part in apoptosis at multiple levels if not in noncanonical ignored cascades (supplemental Figure 5).
Discussion
In this work, we demonstrate that in B-CLL cells, procasp8 is a substrate for Lyn, the SFK that has been found to be overexpressed and to exhibit high constitutive activity as a component of an aberrant cytosolic multi-protein complex. Its phosphorylation occurs at Tyr380 and results in down-regulation of casp8 proapoptotic function; this condition contributes to the defective apoptosis that is a hallmark of this disease. This is the first evidence of how Lyn’s dysregulated expression, activity, and localization mediate resistance to apoptosis in B-CLL by affecting the phosphorylation status of a specific substrate target. Casp8 is a member of the family of cysteine proteases termed caspases, which not only initiate the so-called extrinsic apoptosis pathway but also take part in other numerous nonapoptotic functions, such as cell adhesion, migration, differentiation, and survival.28-35 The structure of procasp-8 consists of: 1) an N-terminal tandem DED, the prodomain, which mediates homophilic interactions with another molecule of procasp8 to form the active dimer, or with regulators and/or adaptor proteins such as cFLIP and FAS-associated death domain, respectively, all of these taking part in the formation of the DISC; and 2) the 2 C-terminal large and small catalytic subunits, separated by a short linker region harboring Tyr380. To be activated, the cytosolic inactive zymogen procasp8 is recruited to the DISC at the plasma membrane upon ligation of death receptors, undergoing dimerization and becoming a substrate for other adjacent dimers. This results in the removal of the linker region and the prodomain, with the generation of the active casp8 composed of 2 large and 2 small subunits, which can then process downstream targets mediating the execution of apoptosis.
We show that procasp8 occurs as a cytosolic inactive homodimer in B-CLL cells compared with normal B-lymphocytes, where this protease presents as a monomeric cytosolic form. The trigger for dimerization appears to be the phosphorylation of Tyr380, as indicated by the backshift of the molecular weight of procasp8 in accordance with its phosphorylation state assessed by glycerol gradient ultracentrifugation (Figure 4) and by the disassembling of this dimeric form due to dephosphorylation by endogenous phosphatases when no phosphatase inhibitors were used in the early steps of purification (supplemental Figure 2). Interestingly, we did not observe any interaction of procasp8 with Lyn (Figure 4), which is at variance with other reports indicating that members of the Src family associate with the procasp8 DED,34 or with other known ligands such as cFLIP39 or the p85 subunit of PI3K38 (data not shown). In this regard, it can be suggested that Tyr380 phosphorylation promotes homodimerization and inhibition of the caspase activity, with a structural rearrangement that prevents any potential partner from targeting procasp8, resulting in its segregation in the cytosol. We also demonstrate that Tyr380 of procasp8, independently of the IGHV mutational status, is phosphorylated by the pool of Lyn that takes part in the aberrant cytosolic multi-protein complex associated with Hsp90 and characterizing B-CLL cells.15 In fact, phosphorylation of Tyr380, in addition to being abolished by inhibitors with diverse selectivity toward SFKs (PP2, SU6656, and dasatinib), is also suppressed by the use of the Hsp90 inhibitor GA, resulting in the disruption of the aforementioned complex and the drop in Lyn’s cytosolic activity, events that have proved to be a crucial step in igniting apoptosis.15 Indeed, dephosphorylation and activation of procasp8 resulting from such treatments further highlight the critical role of Lyn’s altered function as antiapoptotic factor in B-CLL, with one of its direct downstream targets emerging as a novel player in the pathogenesis of this disease.
The casp8-mediated apoptosis elicited by Lyn inhibition does not require the involvement of the death receptor-mediated cascades, which have been described as mostly silent due to the refractoriness of B-CLL cells to death receptor ligation.41,42 In fact, we note that pharmacological and genetic inhibition of procasp8 drastically reduced PP2/dasatinib-dependent apoptosis in B-CLL cells, confirming that procasp8, as a substrate for the cytosolic pool of Lyn, mediates the maintenance of viable leukemia cells.
The importance of the inhibitory axis Lyn/procasp8 is also stressed by the effects downstream of PP2/dasatinib-dependent casp8 activation, with cleavage of PARP indicating activation of the effector caspase-3 and the release of cyt-c being suggestive of the involvement of casp8 itself in propagating proapoptotic stimuli from the cytosol to mitochondria. It remains to be elucidated how dephosphorylation of Tyr380 induces a fully active conformation of procasp8. Although procasp8 dimerization and inhibition can doubtless be regarded as the direct effect of the aberrant activity of Lyn in B-CLL, it is not clear whether activation of procasp8 following Lyn inhibition is achieved by auto-cleavage or may implicate the direct or indirect participation of other factors sensitive to the decrease in tyrosine phosphorylation.
In this regard, it is worth mentioning that the existence of an unconventional pathway of activation of procasp8 has recently been highlighted, with endoplasmic reticulum (ER)-resident caspase-4 cleaving procasp8 upon ER stress and casp8 acting as a hub in bridging ER stress to the intrinsic apoptotic cascade irrespective of Bid truncation.44
The findings proposed in this report again underline the antiapoptotic function of Lyn as a component of the aberrant cytosolic multi-protein complex in B-CLL, although additional efforts are required to shed light on the role of Lyn’s substrates involved in countering cell death, especially considering the broad spectrum of potential targets as revealed by the B-CLL cell cytosolic pTyr-subproteome. Furthermore, this study also highlights a large heterogeneity in the pTyr background, wherein a number of yet-to-be-identified Lyn-dependent substrates are shared by all B-CLL patients like procasp8, which may explain the characteristics of the aberrant signaling network in B-CLL. In this regard, a large-scale phosphoproteomic analysis and proper bioinformatics tools might help identify novel players downstream of the signalosomes, which account for the differences in the level of BCR tonic activation and the apoptotic rate according to the IGHV mutational status evidenced by other authors.7
Overall, our data again underscore the crucial role of Lyn dysregulation in mediating resistance to apoptosis in the deranged signaling background of B-CLL and lead us to propose procasp8 in its functionally down-regulated form as a novel target for the development of molecules capable of activating apoptosis, opening new perspectives in the treatment of this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by A.I.R.C. (Milan) to G.S., by a grant from Fondazione Berlucchi on “Approccio clinico/biologico ai pazienti con leucemia linfatica cronica,” by Regione Veneto on Chronic Lymphocytic Leukemia, by AIRC Regional Project with Fondazione CARIPARO and CARIVERONA, by FIRB (Rome).
Authorship
Contribution: F.Z. performed the majority of the in vitro research and analyzed the data; M.A.P. performed bioinformatics analysis and some of the in vitro research, and wrote and reviewed the manuscript; L.T. provided clinical patient samples, analyzed the data, and wrote parts of the manuscript; E.T. performed some of the in vitro research; F.F., C.G, V.M., and V.T. provided clinical patient samples and performed some of the in vitro research; M.M. and L.B. performed some of the in vitro research; G.S. provided intellectual input into the lymphocyte studies and reviewed the manuscript; and A.M.B. designed the research, reviewed all of the data, participated in analysis of data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Maria Brunati, Department of Molecular Medicine, University of Padua, Viale G. Colombo 3, 35131 Padua, Italy; e-mail: annamaria.brunati@unipd.it; and Livio Trentin, Department of Medicine, University of Padua, Via Giustiniani 2, 35128 Padua, Italy; e-mail: livio.trentin@unipd.it.
References
Author notes
F.Z. and M.A.P. contributed equally to this work.