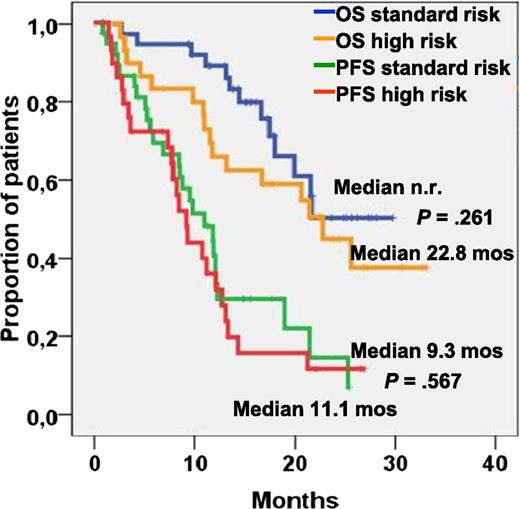
Progression-free and overall survival in patients with standard and high-risk cytogenetics. See Figure 1B in the article by Ludwig et al that begins on page 985.
Progression-free and overall survival in patients with standard and high-risk cytogenetics. See Figure 1B in the article by Ludwig et al that begins on page 985.
Bendamustine, originally termed IMET 3393 and also known as Treakisym, Ribomustin, Levact, Treanda, or SDX-105, is a nitrogen mustard that was developed as a bifunctional molecule with alkylator and antimetabolite properties. Compared with other frequently used alkylating agents, bendamustine induces higher rates of DNA double-strand breaks and is not cross-resistant with melphalan and several other cytotoxic drugs.2
Bendamustine was originally developed in 1963 in East Germany (the former German Democratic Republic) by Ozegowski and Krebs.2 Over a 20-year period, it became highly used in the Eastern Bloc before the fall of the Iron Curtain in 1989. More than 18 000 patients using this compound were studied in the mid 1990s, mostly in Germany. In 2008, bendamustine was approved by the US Food and Drug Administration for the treatment of chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
Over the past decade, there was increasing evidence that bendamustine is also very active in multiple myeloma, and it is currently being tested in a clinical trial for amyloid light-chain amyloidosis (http://clinicaltrials.gov/ct2/show/NCT01222260). A dose-escalation study of single-agent bendamustine up to 100 mg/m2 showed an overall response rate (ORR) of 55%, including minor responses (MRs).3 Pönisch et al performed a phase 1 clinical trial testing the combination of bendamustine, prednisolone, and thalidomide for relapsed or refractory multiple myeloma.4 Fixed doses of bendamustine (60 mg/m2) and prednisolone (100 mg) with escalating doses of thalidomide (50, 100, and 200 mg) achieved a high response rate of 86%, including 14% complete responses. A direct comparison with current data is difficult due to the use of different response criteria without free light-chain assessment. In another phase 1/2 trial, our group established the maximum tolerated dose (MTD) of the combination of bendamustine (75 mg/m2), lenalidomide (10 mg), and dexamethasone (40 mg) with an ORR of 50%. Most of the patients were pretreated with lenalidomide and bortezomib and had a median of 3 prior treatment lines.5 In a different phase 1/2 trial with less heavily pretreated patients, the MTD was not reached using up to 75 mg/m2 of bendamustine and 25 mg lenalidomide with an ORR of 75%.6
The most frequently observed adverse events when combining bendamustine with lenalidomide are neutropenia and prolonged thrombocytopenia. Therefore, a combination of bendamustine with a less hematotoxic agent such as bortezomib provides a very promising approach that will allow the administration of adequate therapeutic doses. Indeed, Pönisch et al conducted a clinical trial using bendamustine, bortezomib, and prednisone and observed a response rate of 69%.7 The Intergroupe Francophone du Myélome presented data obtained with bendamustine, bortezomib, and dexamethasone in elderly patients after 1 line of therapy with responses rates up to 67.1%.8 Berenson et al conducted a phase 1/2 trial of bendamustine (90 mg/m2) in combination with a fixed low dose of bortezomib (1 mg/m2) without corticosteroids in heavily pretreated patients resulting in an ORR of 33% and 48%, including MRs.9
The current study by Ludwig et al1 published in this issue of Blood is the largest prospective phase 2 trial using BBD for relapsed or refractory multiple myeloma. BBD resulted in a very high response rate of 60.9% or 75.9% if MRs were included. Including only patients who received ≥2 cycles of therapy (71 patients), the ORR increased to 67.7% or 84.6% (with MRs). As expected, the response rates were lower in patients pretreated with bortezomib, lenalidomide, or both, in patients exposed to ≥2 prior lines of lenalidomide-based therapy, and in those with relapsed and/or refractory disease to the last treatment regimen. Nevertheless, 5 of the 8 patients who had been pre-exposed to 2 or 3 lines of bortezomib achieved at least a partial response or better, suggesting that the BBD regimen induces meaningful responses even after multiple treatments in relapsed/refractory patients.
Most interestingly, BBD resulted in fast responses, with a time to response and time to best response of 31 days and 111 days, respectively. This is of high clinical relevance, because rapid tumor control usually corresponds with fast improvement of clinical symptoms and is especially important in patients with high tumor burden and aggressive disease. Similar findings have also been reported in studies using bendamustine with lenalidomide and corticosteroids.5,6
Besides the induction of fast and high responses by this regimen, probably the most significant finding of this study is that BBD achieved similar response rates but, even more importantly, comparable progression-free survival and overall survival in patients with or without cytogenetically defined high-risk features (see figure). This suggests that the combination of bortezomib and bendamustine is even more effective than bortezomib alone in overcoming the adverse prognosis of multiple myeloma patients with high-risk cytogenetics.
Pharmacologic studies have shown that bendamustine can be given safely in patients with renal failure. A study on a small group of patients with stage IV or V renal impairment showed no unexpected toxicity and a response rate of 55% when a single dose of 120 mg/m2 bendamustine was given in combination with daily 100 mg thalidomide and weekly dexamethasone for the first 3 weeks of a 4-week cycle.10 This suggests that the combination of bendamustine, bortezomib, and dexamethasone provides a very effective and safe option in high-risk patients with renal failure and adverse cytogenetics.
In summary, the study by Ludwig and colleagues shows that BBD
is a highly effective regimen in heavily pretreated patients;
overcomes adverse cytogenetics;
induces very fast remissions;
can be given in renal failure; and
is a safe combination with a well-defined toxicity profile.
Based on the excellent response data, especially in patients with adverse cytogenetics, and a favorable toxicity profile, combinations of bendamustine with second-class proteasome inhibitors such as carfilzomib are promising and currently tested in the first- and second-line setting (http://clinicaltrials.gov/ct2/show/NCT02002598).
Conflict-of-interest disclosure: The author declares no competing financial interests.