Today human leukocyte antigen-haploidentical transplantation is a feasible option for patients with high-risk acute leukemia who do not have matched donors. Whether it is T-cell replete or T-cell depleted, it is still, however, associated with issues of transplant-related mortality and posttransplant leukemia relapse. After reports that adoptive immunotherapy with T-regulatory cells controls the alloreactivity of conventional T lymphocytes in animal models, tomorrow’s world of haploidentical transplantation will focus on new “designed” grafts. They will contain an appropriate ratio of conventional T lymphocytes and T-regulatory cells, natural killer cells, γ δ T cells, and other accessory cells. Preliminary results of ongoing clinical trials show the approach is feasible. It is associated with better immune reconstitution and a quite powerful graft-versus-leukemia effect with a low incidence of graft-versus-host disease and no need for posttransplant pharmacological prophylaxis. Future strategies will focus on enhancing the clinical benefit of T-regulatory cells by increasing their number and strengthening their function.
Introduction
Hematopoietic stem cell transplantation from a full haplotype-mismatched family member (haplo-HSCT) has come a long way since it was pioneered in the 1980s in severe combined immunodeficiency patients.1 Although indications for its use in patients with acute leukemia (AL) have never been properly defined, it is today a feasible option for patients at high risk of relapse who do not have human leukocyte antigen (HLA)-matched donors.2 Advantages include immediate donor availability, particularly for candidates in urgent need of transplantation; the opportunity to select the best donor from a panel of family members on the basis of age, cytomegalovirus (CMV) status, and natural killer (NK) cell alloreactivity; and easy access to donor-derived cellular therapies after HSCT, if required.
The road to full maturity of haplo-HSCT was beset by clinical problems. Until the 1990s, haplo-HSCT was associated with a high incidence of graft rejection in T-cell–depleted transplants and severe graft-versus-host disease (GVHD) in unmanipulated transplants because of the high frequency of T cells that recognized major class I or II HLA disparities between donor and recipient.2 To overcome these problems, 2 approaches were developed: a megadose of T-cell–depleted hematopoietic progenitor cells without any posttransplant immunosuppression and unmanipulated grafts with innovative pharmacological immunosuppression for GVHD prophylaxis.
T-cell–depleted haplo-HSCT
Megadose T-cell–depleted transplants were shown to overcome graft rejection in mouse models of mismatched transplantation.3 Clinical success (ie, primary full donor type engraftment in >95% of AL patients and very little acute GVHD [aGVHD] and chronic GVHD [cGVHD]) was achieved with a graft containing a megadose (10 × 106/kg) of negatively4 or positively5 immunoselected CD34+ cells. Conditioning included total body irradiation (TBI), thiotepa, fludarabine, and antithymocyte globulin (ATG). No posttransplant pharmacological immunosuppression was given, even though ATG in the conditioning exerted additional T-cell depletion in vivo.5,6 Two remarkable observations emerged. Engraftment was promoted by the megadose of CD34+ cells, because they suppressed cytotoxic T-lymphocyte precursors directed against their own antigens (veto effect).7 A powerful graft-versus-leukemia (GvL) effect in the absence of GVHD was induced in acute myeloid leukemia (AML) patients by posttransplant generation of the donor vs recipient alloreactive NK cell repertoire.8,-10 Human NK cell function is regulated by clonally distributed inhibitory receptors termed killer cell immunoglobulin-like receptors (KIRs) that recognize HLA class I allele groups (KIR ligands). Only NK cells that express inhibitory KIRs for self HLA ligands become fully functional (or licensed/educated). When confronted with an allogeneic target, such as after KIR ligand mismatched haplo-HSCT, educated donor NK cells, which have as their only inhibitory receptor for donor HLA a KIR that does not recognize recipient HLA, sense the missing expression of the ligand and mediate alloreactions (“missing self” recognition).
With more than 15 years of follow up, results showed a 43% disease-free survival (DFS) in AML and 30% in acute lymphoblastic leukemia (ALL) patients.5,6,11 In the Perugia series of high-risk AML patients, NK cell alloreactivity (which is potentially available for almost 50% of patients) was associated with a significantly lower relapse rate (3% vs 47%; P < .003) and better event-free survival (EFS) (67% vs 18%; P = .02) when patients were transplanted in remission and 34% EFS when they were transplanted in relapse. Similar benefits were observed in children with ALL but not in adults.12,,-15
One major issue is slow posttransplant immune recovery due to the few residual T lymphocytes in the graft and in vivo ATG-linked T-cell depletion. Thus, T-cell–depleted haplo-transplant recipients remain susceptible to life-threatening opportunistic infections for several months. Another drawback is the cumulative incidence of posttransplant relapse, which is still >30% in high-risk ALL patients and in those with AML who were not transplanted from NK alloreactive donors.
To improve posttransplant immune reconstitution and reduce transplant-related mortality (TRM), adoptive immunotherapies were explored with pathogen-specific T lymphocytes16,,-19 or broad repertoire T cells that were depleted of alloreactive T cells20 or engineered with a suicide gene.21,22 The former were clearly unsuitable for prophylaxis, and the latter could not be infused in sufficient numbers because of the high risk of GVHD. Furthermore, both approaches were cumbersome and required good-manufacturing-practices facilities.
In an innovative approach, Handgretinger’s group in Tubingen23 depleted the leukapheresis product of only T-cell receptor (TCR) α/β + (TCRαβ+) cells, thus retaining large numbers of effector cells such as TCRγδ+ T cells and NK cells. TCRγδ+ T cells combine conventional adaptive features with direct, rapid responses against sterile stresses and many pathogens.24,25 They participated in the anti-CMV response,26,-28 particularly when conventional adoptive immune mechanisms were insufficient or absent, that is, in the early period of posttransplant immune recovery.29 They are not expected to initiate GVHD, because they do not recognize specific processed peptide antigens as presented on major histocompatibility complex (MHC) molecules. Furthermore, they appear to exert antitumor30,31 and antileukemic activity,32 because they directly recognized stress-induced self-antigens expressed by malignant cells. In this approach, the GvL effect relies mainly on TCRγδ+ T cells, beause, as in standard T-cell–depleted HSCT, very few conventional T cells (Tcons) are infused.
To remove TCRαβ+ T lymphocytes, a biotinylated anti-TCR αβ antibody was employed, followed by an antibiotin antibody conjugated to magnetic microbeads. A 4.7 log depletion was achieved, with a median of 14 × 103/kg infused TCRαβ+ T lymphocytes. CD19+ B lymphocytes were also immunodepleted to prevent posttransplant Epstein-Barr virus–associated lymphoproliferative disorders.33 Recovery of CD34+ hematopoietic progenitor cells (74%) was similar to CD34+ enrichment procedures. Before transplantation with TCRαβ+/CD19+ depleted grafts, children in Tubingen received a chemotherapy-based conditioning, whereas Locatelli and coworkers33 in Rome administered a TBI-based conditioning. In both cohorts, no posttransplant GVHD prophylaxis was given, although anti-T antibodies (OKT3 or ATG) in the conditioning exerted additional in vivo T-cell depletion. Engraftment was very rapid in all patients. Few had acute grade 1 and 2 GVHD, and none developed cGVHD, confirming that TCRγδ+ T cells do not cause GVHD. Immune reconstitution was fast in these children. A longer follow up and more patients are required to assess the posttransplant relapse rate.
T-cell–replete haplo-HSCT
T-cell–replete haplo-HSCT has recently become an attractive alternative modality. Interest in it was reawakened by new transplant strategies for GVHD prophylaxis, such as posttransplant, high-dose cyclophosphamide or granulocyte colony-stimulating factor–primed grafts in combination with other immunosuppressive agents. Furthermore, it does not necessitate any ex vivo graft manipulation.
In the 1970s George Santos demonstrated that a short course of high-dose cyclophosphamide soon after bone marrow transplant in rodents targeted activated donor and host alloreactive T cells.34 Support for high-dose cyclophosphamide after clinical HSCT derived from newer data showing it was not toxic to hematopoietic stem cells, because they highly express aldehyde dehydrogenase, the detoxifying enzyme.35,36
The John Hopkins and Fred Hutchinson Cancer Research Center groups assessed the efficacy of cyclophosphamide (50 mg/kg days +3, +4), mycophenolate mofetil, and tacrolimus for posttransplant GVHD prophylaxis in 210 AL patients who received a non-myeloablative conditioning.37 Engraftment was sustained in 87%; grade 2-4 aGVHD occurred in 27%, grade 3-4 in 5%, and cGVHD in 15%. The cumulative incidences of relapse and nonrelapse mortality were 55% and 18%, respectively. Three-year overall survival and EFS were 41% and 32%, respectively. Unfortunately, a high relapse rate counterbalanced the relatively low TRM. In patients at very high risk of leukemia relapse, the nonmyeloablative conditioning regimen may not have been intense enough to achieve sufficient leukemia debulking; the GvL effect may even have been weakened by the cyclophospamide itself and other immunosuppressive agents, because, in blocking T-cell alloreactivity, they can also inhibit the T-cell–related GvL activity.
Since then, several single and multicenter phase II trials confirmed that high doses of cyclophosphamide within a narrow posttransplant time frame were associated with low incidences of GVHD and a relatively low TRM. Bashey et al38 reported that outcomes were similar after haplo-HSCT with posttransplant cyclophosphamide, HLA-matched sibling, and matched unrelated donor (MUD) transplants. At 6 months cumulative incidences of grades 3-4 aGVHD were 11%, 8%, and 11%, respectively (P not significant); extensive cGVHD occurred in 38%, 54%, and 54% of patients, respectively. The 2-year cumulative incidences of nonrelapse mortality were 7%, 13%, and 16%, respectively, and relapse rates were 33%, 34%, and 34%. Probabilities of DFS were 60%, 53%, and 52%, respectively.
To date, no randomized clinical trials have compared outcomes after T-cell–depleted and T-cell–replete haplo-HSCT. In a nonrandomized study, Ciurea et al39 compared outcomes after T-cell–replete or T-cell–depleted haplo-grafts in a relatively small cohort of consecutive patients, most of whom had AL. All patients received a chemotherapy-based myeloablative conditioning: melphalan, fludarabine, and thiotepa (plus ATG only in the T-cell–depleted group). Only the T-cell–replete group received posttransplant GVHD prophylaxis with cyclophosphamide, tacrolimus, and mycophenolate. Engraftment was 94% vs 81% in the T-cell–depleted group (not significant) and TRM at 1 year was 16% vs 42% (P = .02). The cumulative incidence of grade 2-4 aGVHD was 20% vs 11%, and cGVHD was 7% vs 18% (P = .03). The T-cell–replete group had better T-cell subset reconstitution and fewer infections. Actuarial progression-free survival rates at 1 year posttransplant were 50% vs 21% (P = .02).
Before any definitive conclusions can be drawn, a randomized study is required. It needs to take into account that, in T-cell–depleted haplo-HSCT, a TBI-based conditioning is reported to be associated with best outcomes and engraftment rates approaching 100%. Unfortunately, the chemotherapy-based conditioning used in the Ciurea et al39 study was beset by 20% graft failure.
In a different approach to downmodulate donor T-cell alloreactivity, grafts consisted of “G-CSF–primed” bone marrow and peripheral blood40 or only bone marrow.41 Patients received a myeloablative conditioning and intensive posttransplant immunosuppression, for example, ATG, cyclosporine, methotrexate, mycophenolate mophetyl, and anti-CD25 antibody.41
In 250 AL patients (89 at high risk), the Huang group40 in Beijing reported nearly 100% full-donor engraftment. The cumulative incidences of grade 2-4 and 3-4 aGVHD were 45.8% and 13.4%, respectively, with 53.9% cGVHD, which was extensive in 22.6%. The 3-year probabilities of DFS were, respectively, 70.7% and 55.9% in standard- and high-risk AML patients and 59.7% and 24.8% in ALL.
The Di Bartolomeo et al41 study also yielded promising results in terms of engraftment, incidence of GVHD, and survival. The cumulative incidences of grade 2-4 and grade 3-4 aGVHD at 100 days were 24% ± 0.2% and 5% ± 0.6%, respectively. TRM was 34%, and the overall cumulative incidence of relapse was 21% ± 0.2% at 1 year and 28% ± 0.3% at 5 years.
In conclusion, whether haplo-HSCT is T-cell depleted or not, several trials (but not all) reported survival rates in patients with high-risk AL that appeared to be in the range of those after well-matched MUD HSCT. Like MUD and HLA-sibling HSCTs, posttransplant relapse rates are ∼25% to 30% (unless NK alloreactivity is exploited), which is hardly satisfactory. Furthermore, both modalities are still beset with their original drawbacks: slow posttransplant immune reconstitution in patients who receive T-cell–depleted transplants and acute and cGVHD in those who opt for T-cell–replete grafts.
Designing haplo-grafts to separate the GVL effect from GVHD and improve immune reconstitution
In the setting of allogeneic HSCT, conventional CD4+ and CD8+ T cells (Tcons) in the donor grafts have long been recognized as a double-edged sword. They facilitate engraftment, accelerate immune reconstitution,42 and contribute to the elimination of residual disease (GvL effect)43,44 by exploiting histocompatibility differences between donor and recipient. GvL induction by conventional HSCT is, on the other hand, quite a rudimentary form of leukemia immunotherapy, because unselected T cells react against a multitude of host alloantigens and mediate immune destruction of host tissues (GVHD). Prophylaxis by means of posttransplant pharmacological immunosuppression is immunologically nonspecific, only partially successful, and may even compromise the T-cell–induced GvL effect. Because GVHD and GvL both derive from alloreactivity, how can alloreactive T cells be manipulated to spare normal cells yet kill leukemic cells? Until recently, attempts to identify and separate these specific immune effector mechanisms were largely unsuccessful.
In the search for an alternative strategy to prevent GVHD, attention focused on a thymic-derived CD4+CD25+ FoxP3+ T-cell subpopulation that plays a physiological role in maintaining immunological self-tolerance and immune homeostasis.45,46 In several murine models of bone marrow transplantation across MHC class I and II barriers, lethal GVHD was suppressed when freshly isolated47,-49 or ex vivo-expanded polyclonal50 or recipient-type51 FoxP3+ regulatory T cells (Tregs) were coinfused with conventional T lymphocytes (Tcons). Critical for Treg survival and activation after infusion is their recognition of alloantigen peptides, which recipient antigen presenting cells (APCs) present on MHC class II molecules.52 Tracking Treg in vivo dynamics showed that infusion of freshly isolated polyclonal Tregs was followed by activation and robust expansion of alloantigen-specific Tregs in lymph nodes and then by their migration to GVHD target tissues (skin, gut, liver, lung).48 During their effector phase, Tregs markedly reduced early alloreactive T-cell proliferation in lymph nodes and perhaps in nonlymphoid tissues48 by means of several mechanisms,53 for example, via interaction with APCs in priming sites as indicated by intravital microscopy analysis.48 Moreover, Tawara et al54 demonstrated that host APC alloantigen presentation to donor Tregs is necessary and sufficient for Treg-mediated suppression of GVHD.
Tregs specifically suppressed alloreactive T cells but allowed functional immune system reconstitution. Adoptive transfer of Tregs prevented GVHD-induced damage of the thymic and secondary lymphoid microenviroment, accelerated donor lymphoid expansion of a diverse TCR V β repertoire, and improved immune reconstitution, thus protecting mice from lethal CMV infection.49 Gaidot et al55 studied the effects of recipient-specific Tregs on immune reconstitution in a mouse model of HSCT that excluded thymic output. Immune reconstitution was thus derived, as in human adults, only from mature T cells within the graft. Adoptive immunotherapy with Tregs prevented GVHD and improved immune reconstitution in terms of cell numbers, activation phenotype, and cytokine production. Because immune function was preserved in vivo, as demonstrated by vaccinia infection and third-party skin-graft rejection models, Treg immunosuppression appeared to be relatively specific for GVHD prevention.
In the clinical application of Tregs in HSCT, one major concern is potential suppression of immune-mediated GvL responses, in light of reports indicating that Tregs may contribute to a defective immune response against solid tumors56,57 and hematological malignancies.58,59 In mice, Tregs accumulated in leukemic sites, impeded proliferation of adoptively transferred anti-AML reactive cytotoxic T lymphocytes, and consequently cytolysis.60 Treg suppression was reversed by interleukin-2 diphtheria toxin, which depleted Tregs expressing CD25 and resulted in temporary tumor regression associated with increased cytotoxic T lymphocytes at tumor sites.61 On the other hand, in mismatched transplant mouse models, adoptive immunotherapy with Tregs and Tcons protected mice from GVHD without impairing Tcon control of neoplastic expansion.62,63 Similarly, adoptive transfer of Tregs and Tcons eradicated leukemia without GVHD in mice engrafted with human myeloid primary leukemia or AML or ALL cell lines.64
The underlying mechanisms are unclear. Edinger et al63 showed that Tregs prevent GVHD by blocking Tcon alloantigen-driven expansion but not function. Indeed, Tregs did not inhibit the cotransplanted Tcon activation and killing of leukemia and lymphoma cells in vitro and in vivo. Thus, in this setting, GvL activity appears to rely mainly on activation of alloantigen specific Tcons rather than expansion.63,65
Another interesting hypothesis is that Treg-mediated preservation of the GvL could be related to still unknown mechanisms that limit graft-versus-host reactions to mainly hematopoietic cells, which are indeed more sensitive to alloreactive T cells than other cell types. Ranking is as follows: hematolymphoid cells > thymic epithelial cells > epithelial cells from the gut, liver, and skin > other organs that are spared by GVHD (eg, heart and kidney).66
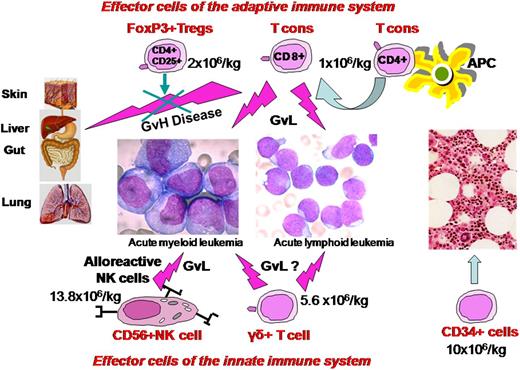
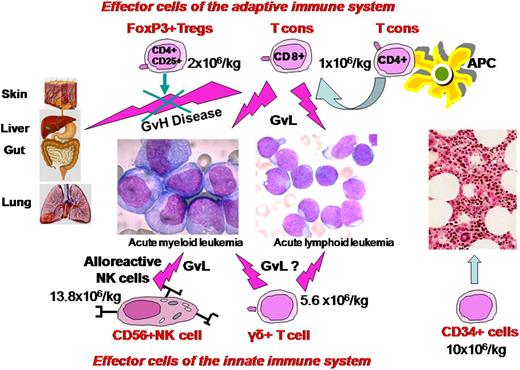
The platform of T-cell–depleted haplo-HSCT without any posttransplant immunosuppression together with these insights from animal models prompted us to focus on adoptive immunotherapy with a high number of broad repertoire T cells, coinfused with freshly isolated CD4+CD25+ FOXP3+ Tregs. The main obstacle to clinical application was obtaining a suitable number of Tregs from peripheral blood under good-manufacturing-practices conditions. Using a fully automated closed system, we managed to reliably immunoselect 2-4 × 106/kg CD4+CD25+ Tregs from a leukapheresis product, obtaining in the final fraction 90% FoxP3+ cells.67 High-risk AL patients received a conditioning regimen that included 8Gy TBI, thiotepa, cyclophosphamide, and fludarabine.68 The conditioning was subsequently modified by substituting anti-T antibodies (alemtuzumab or thymoglobulin) for cyclophosphamide to reduce extrahematological toxicity.64 On day −4 patients were infused with freshly isolated Tregs (median 2 × 106/kg) followed by a megadose of positively immunoselected CD34+ cells (median 10 × 106/kg) and conventional T lymphocytes (median 1 × 106/kg) on day 0. No posttransplant immunosuppression was given. A 4-day gap between Treg and Tcon infusions was chosen, because murine models indicated that early administration of Tregs provided the best protection against GVHD due to their in vivo proliferation in the proinflammatory postconditioning environment of a mismatched, T-cell–depleted recipient. In fact, Nguyen et al48 reported that if Tregs were administered 2 days before Tcons, mice were protected from GVHD even with a much lower Treg/Tcon ratio than usual, that is, 1:10 instead of 1:1 per animal.
Only 6/43 evaluable patients developed grade II-IV aGVHD. No patient had chronic GVHD, confirming that in humans as in animal models, early adoptive transfer of naturally occurring donor Tregs made administration of a high dose of mature Tcons feasible and kept the incidence of GVHD very low in the absence of any posttransplant pharmacological immunosuppression.
The pattern of posttransplant immune reconstitution was markedly different from standard T-cell–depleted haplo-HSCT.68 Coinfused Tcons developed a wide T-cell repertoire, with CD4+ and CD8 counts reaching a 50/μL median on days 33 and 27, respectively; and 200/μL on days 67 and 48. High frequencies of pathogen-specific CD4+ and CD8+ T-cell precursors were detected as early as 2 months posttransplant and the incidence of CMV disease decreased markedly. Vaccination against pandemic influenza with MF59-H1N1 California resulted in strong antiviral protection, showing that patients had become immunologically competent 3-4 months after transplant. In patients transplanted from potentially NK alloreactive donors, adoptive transfer of Tregs did not impair NK cell posttransplant regeneration/maturation. It was faster than in standard T-cell–depleted haplo-HSCT, with enhanced donor vs recipient alloreactive NK cell repertoires against KIR-ligand mismatched targets.
Results in our 12 ALL and 33 AML patients (20 without NK alloreactive donors) show a powerful GvL activity was exerted against both myeloid and lymphoid leukemic cells without being associated with GVHD. Only 2 AML patients who were transplanted from non-NK alloreactive donors have relapsed to date. The cumulative incidence of posttransplant leukemia relapse was 0.06 at a median follow up of 46 months, which is extremely low considering these patients were at high risk of relapse according to cytogenetics, molecular markers, and disease stage at transplant.64 In our view, the relatively high number of infused conventional T lymphocytes was able to exert a powerful antileukemic effect due to lack of posttransplant pharmacological immunosuppression. They could also have masked any synergistic effect exerted by alloreactive NK cells.
Thus, in humans as in animal models, coinfusion of CD4+CD25+FoxP3+Tregs and Tcons protected recipients against GVHD without impairing the Tcons’ related GvL effect.
An interesting step forward from grafts with a high Tcon content under the protective Treg umbrella are grafts that also contain NK cells, TCRγδ+ T cells, and other accessory cells (Figure 1). Infusion of high numbers of NK cells would be expected to provide an antileukemic effect immediately after administration and afford better protection against infections. TCRγδ+ T cells would improve resistance to pathogens in the critical early posttransplant phase and hopefully contribute to GvL effect. Switching graft processing techniques from CD34+ enrichment to TCRαβ+/CD19+ depletion, followed by the addition of naturally occurring Tregs and Tcons, is a simple way to achieve this innovative type of graft. We are currently conducting a pilot study in patients with high-risk AL. After a chemotherapy-based conditioning, they receive a graft consisting of freshly isolated donor Tregs (2 × 106/kg) and Tcons (1 × 106/kg) in a 2:1 ratio followed by 10.2 (7.0-16.1) × 106/kg CD34+ hematopoietic progenitor cells, 12.9 (0.00-63.9) × 104/kg TCRαβ+cells, 13.8(1.9-32.4) × 106/kg CD56+NK cells, and 5.6 (1.4-15.2) × 106/kg TCR γδ+T cells. Preliminary results in 10 patients with a median age of 55 years show this approach is feasible, with a high rate of engraftment and only 1 case of aGVHD.
Composition and mechanism of action of a new “designed” graft. It contains a megadose of CD34+ cells that are depleted of TCRαβ+/CD19+ cells plus adoptive immunotherapy with FoxP3Tregs and Tcons in an established ratio of 2:1. Besides the GVL effect, Tcons, NK cells, and TCRγδ+ T cells hasten immune reconstitution.
Composition and mechanism of action of a new “designed” graft. It contains a megadose of CD34+ cells that are depleted of TCRαβ+/CD19+ cells plus adoptive immunotherapy with FoxP3Tregs and Tcons in an established ratio of 2:1. Besides the GVL effect, Tcons, NK cells, and TCRγδ+ T cells hasten immune reconstitution.
An alternative to adoptive immunotherapy with freshly isolated Tregs is ex vivo expanded Tregs, which offer the joint advantages of more Tregs than can be collected from a leukapheresis product and the chance to transplant more Tcons.69,70
Using anti-CD3/anti-CD28 antibody coated Dynabeads to expand polyclonal Tregs ex vivo, Brunstein et al71 evaluated Treg infusion as supplemental GVHD prophylaxis in AL patients. They received double-unit unrelated donor umbilical cord blood transplantation followed by infusion of third-party unrelated cord blood Tregs. Mycophenolate mofetil and sirolimus were also given as posttransplant prophylaxis against GVHD. Compared with historical controls, the incidence of grade 2 to 4 GVHD decreased significantly, whereas the incidence of posttransplant leukemia relapse remained unchanged.
Several other approaches are under preclinical investigation. Polyclonal naïve CD4+CD25+CD45RA+ Tregs are the strongest immunosuppressive natural Tregs and the most stable in culture, apparently making them ideal candidates for ex vivo expansion and adoptive immunotherapy.72 Treg surface markers such as LAG-3, MHC class II, and CD62L could be helpful in Treg selection to better exploit their suppressive function.73,,,-77 Studies are needed to clarify the impact of ex vivo expanded “third-party” Tregs in the prevention of GVHD, because HLA identity between Tregs and Tcons is apparently required for maximum immunosuppression.78 Treg antigen specificity also merits investigation, as in vitro primed antigen specific Tregs exerted a strong selective and specific activity.70,79,80 Thanks to emerging techniques for TCR, repertoire sequencing libraries of antigens that are most involved in inducing GVHD can now be created, thus potentially providing a platform to build individual specific Tregs.
In conclusion, we are confident we are now within sight of the Holy Grail of haplo-transplant. Years of research have taken us from haplo-grafts containing only a megadose of highly purified CD34+ cells and few T cells that were downright antagonized in vivo by ATG to new “designed” grafts containing all blood cell types as well as enough Tregs cells to control T-cell alloreactivity. This innovative use of Treg-Tcon adoptive immunotherapy provides not only a powerful T-cell–dependent GvL effect in the absence of GVHD but also expansion of donor T cells that provide long-term immunity. The decades-long struggle to enhance the GvL effect of haplo-HSCT while suppressing GVHD may finally be coming to an end.
Acknowledgments
We thank Dr Geraldine Anne Boyd for her help. This paper is dedicated to the late Prof Antonio Tabilio, who made such a fundamental contribution to the development of haploidentical transplantation.
Authorship
M.F.M. drafted the paper; M.F.M., M.D.I., L.R., and F.F. designed research and oversaw the results; B.F., Y.R., F.A., and A.V. contributed to the design and interpretation of the study; and F.F., A.C., A.T., and A.P. provided clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mauro Di Ianni, Hematology Section, Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; e-mail: mauro.diianni@cc.univaq.it.