Leukemia in pregnancy remains a challenging therapeutic prospect. The prevalence is low at ∼1 in 10 000 pregnancies, and as a result data are limited to small retrospective series and case reports, rendering evidence-based recommendations for management strategies difficult. The management of the leukemias in pregnancy requires close collaboration with obstetric and neonatology colleagues as both the maternal and fetal outcomes must be taken into consideration. The decision to introduce or delay chemotherapy must be balanced against the impact on maternal and fetal survival and morbidity. Invariably, acute leukemia diagnosed in the first trimester necessitates intensive chemotherapy that is likely to induce fetal malformations. As delaying treatment in this situation is usually inappropriate, counseling with regard to termination of pregnancy is often essential. For chronic disease and acute leukemia diagnosed after the second trimester, therapeutic termination of the pregnancy is not inevitable and often, standard management approaches similar to those in nongravid patients can be used. Here, the management of the acute and chronic leukemias will be addressed.
Introduction
Prior to the introduction of national and international cancer registry databases, the evidence base for the management of leukemia in pregnancy was largely restricted to retrospective case reports. Conclusions regarding the teratogenicity of individual chemotherapeutic agents therefore should be interpreted with the understanding that the data are limited and collected from a heterogeneous group of patients over a prolonged period of time. The mother should be counseled with regard to the risks of proceeding with the pregnancy, together with the potential side effects and impact of therapy on the neonate. Of course, the foregoing may be much influenced by factors such as religious/ethnic beliefs and whether the ensuing treatment, such as allogeneic stem cell transplantation, may result in permanent infertility. The effects on the fetus, not only of the cytotoxic agents but also of the additional supportive medication in the form of antibiotic and antifungal therapy that might be required during chemotherapy, must be considered and protocols evaluated according to their impact on gonadal function. Due to the complex nature of the therapeutic approach in pregnancy, early involvement of obstetric and neonatology colleagues is fundamental to ensure that the balance between maternal and fetal outcome can be carefully considered. Wherever possible a plan should be instituted to schedule delivery so as to avoid maternal and fetal cytopenia as a result of therapy. Advice regarding breastfeeding should be provided in the context of continuing therapy postpartum.
Acute leukemia
The presentation of acute leukemia in pregnancy is broadly similar to the nonpregnant state, although pregnancy may obscure some of the clinical signs. The majority of the leukemias diagnosed in pregnancy are acute and predominantly myeloid as the incidence of acute lymphoblastic leukemia is more common in childhood and adolescence. If the disease is left untreated it will likely result in maternal and fetal mortality1 and a decision to delay start of induction chemotherapy negatively impacts on the likelihood of remission.2 Data suggest that maternal outcomes for acute myeloid leukemia (AML) following chemotherapy are analogous to nonpregnant patients, and consequently delay in commencing treatment is to be avoided.3 The therapeutic approach to the management of acute leukemias in pregnancy regardless of subtype is generally similar. Although a bone marrow aspirate and trephine biopsy may be performed safely in pregnancy,4 these can be avoided if confirmation is clearly possible by means of peripheral blood microscopy, flow cytometry and molecular analysis.
AML
AML occurs more frequently with advancing age and as expected, there is a greater body of data regarding the therapeutic approach for AML in pregnancy. Due to the aggressive nature of the disease, treatment cannot be delayed indefinitely and the balance between the consequences of intensive chemotherapy on both fetus and mother, as well as the effect of postponing treatment on the mother, must be carefully evaluated. Clinical data suggest a similar prognosis for women treated during pregnancy compared with nonpregnant patients,5 despite the underlying malignant disease adversely affecting perinatal outcome. The long-term effects and choice of chemotherapy should also be considered with regard to impairing future fertility, although most modern acute leukemia remission-induction regimens do not induce sterility.
Pregnancy may affect drug metabolism as a result of an altered distribution due to an often greatly increased plasma volume, the presence of the amniotic sac creating a third space, and changes in both hepatic and renal metabolism.6 It is noteworthy that many of the cytotoxic agents have a molecular weight of <400 kDa and can therefore cross the placenta.7 Much of the outcome data are derived from single-agent therapy in animal models, but as the doses used in humans are usually lower the prediction of outcomes can be inaccurate. In practice, the risk of fetal malformation appears lower than might be predicted from animal data but is increased when additional agents are used in combination.8 Lack of clear scientific-based guidelines, due to the lack of pharmacokinetic studies in pregnant women receiving chemotherapy, has meant that in general standard weight-based drug doses have been used which are then adjusted according to ongoing weight gain.8
Existing protocols to treat AML traditionally include remission-induction chemotherapy with the anti-metabolite cytarabine and an anthracycline (often daunorubicin). Both agents are well recognized to cause fetal abnormalities.9,-11 Theoretically, maternal nutritional deficiencies, caused by the underlying disease or chemotherapy-induced anorexia, may also impact on fetal growth and birthweight.8 Antimetabolites appear to be the most teratogenic in comparison with other chemotherapeutic drugs. Cytarabine has been associated with limb deformities (Table 11,3,8,-10,12,,,-16 ).8,12 The incidence of fetal damage with daunorubicin is similar to doxorubicin and is viewed as relatively safer during pregnancy although concerns have been raised with regard to fetal cardiotoxicity16,17 (Table 1). It may be that anthracyclines are less liable to cross the placenta and affect the fetus due to their somewhat larger molecular weight (>500 kDa), hydrophilic molecular properties, and also that they are substrates for placental P-glycoprotein18 limiting fetal exposure. Experience with the topoisomerase inhibitor etoposide is limited and as a consequence is not recommended. More recently, embryonic exposure to topoisomerase II inhibitors has been linked to genomic instability and mixed-lineage leukemia rearrangement.19 Modern management of AML can also include targeted therapy with monoclonal antibodies such as the anti-CD33 monoclonal antibody conjugated to calicheamicin, (gemtuzumab ozogamicin [Mylotarg]), as well as with multikinase inhibitors with activity against receptor tyrosine kinases. Information regarding their effects on fetal development is lacking.
Acute leukemia in pregnancy
| . | First trimester . | Outcome . | Second/third trimester . | Outcome . |
|---|---|---|---|---|
| AML: overall outcome | ||||
| N = 898 | n = 20 | CA, n = 3; TOP, n = 1; Normal development, n = 16 (16 y FU) | Second trimester, n = 54 | Live births, n = 46 (IUGR n = 6) FD, n = 8 (IUFD, n = 6; SB, n = 1; TA for CA, n = 1) |
| N = 311 | n = 6 | SA, n = 2; TA, n = 4 | Second trimester, n = 10 | TA, n = 5; SA, n = 1; Live birth, n = 4 (FTND, n = 3; LSCS, n = 1; Prem LCSC, n = 1) |
| Third trimester, n = 15 | Live birth, n = 15 (FT, n = 12; Prem, n = 3) Maternal outcome: toxic death, n = 1; refractory disease, n = 1 | |||
| APL: outcome following ATRA | ||||
| Shapira et al12 | Up to 85% CA | n = 15 | Normal development | |
| Late first trimester, n = 415 | Normal development | |||
| Siu et al13 | — | — | n = 3 | Transient CM, n = 1 Reversible fetal arrhythmia, n = 2 |
| Culligan et al3 | — | n = 1 | LSCS at 30 wk, n = 1 | |
| — | n = 1 (+idarubicin) | Placental abruption and IUFD, n = 1 | ||
| Consoli et al14 | — | — | n = 1 | SVD, Prem, n = 1 (Maternal outcome: postpartum death due to ATRA syndrome) |
| ALL: overall outcome | ||||
| Cardonick8 (N = 60) | n = 38 | No abnormalities, n = 32 | Second trimester, n = 14 | FP, n = 2; IUFD, n = 1; FD, n = 1; CM (transient), n = 1 |
| CA, n = 2; SA, n = 1; Maternal death, n = 1; IUGR, n = 3 | Third trimester, n = 8 | Normal development, n = 8 | ||
| Drugs used in induction-consolidation therapy | ||||
| Cytarabine (alone or in combination) | n = 48,12 | Limb deformities, n = 4 | ||
| n = 898 (all stages of pregnancy) | Cytopenia, n = 5; IUFD, n = 6; IUGR, n = 12; FD due to sepsis, n = 2 | |||
| n = 5210 | Normal delivery, n = 26; IUFD, n = 6; Prem, n = 6; CA, n = 5 IUGR, n = 3; FP, n = 3 | |||
| Anthracyclines | n = 2916 (all stages of pregnancy) | No myocardial damage | ||
| n = 319 | CA, n = 3 | n = 103/n = 26 | CA, n = 2; CM, n = 3 (1 lethal case) | |
| Daunorubicin10 | n = 34 | Normal, n = 15; IUFD, n = 6; Prem, n = 6; CA, n = 2; IUGR = 3; FP, n = 3 | ||
| Idarubicin9 | n = 3 | Reversible CM, n = 2; lethal CM, n = 1 | ||
| Methotrexate | n = 238 | No abnormalities | n = 19 | No abnormalities |
| >10 mg/wk12 | Increased Misc; low birth weight; pancytopenia | Facial and skeletal abnormalities, aminopterin syndrome |
| . | First trimester . | Outcome . | Second/third trimester . | Outcome . |
|---|---|---|---|---|
| AML: overall outcome | ||||
| N = 898 | n = 20 | CA, n = 3; TOP, n = 1; Normal development, n = 16 (16 y FU) | Second trimester, n = 54 | Live births, n = 46 (IUGR n = 6) FD, n = 8 (IUFD, n = 6; SB, n = 1; TA for CA, n = 1) |
| N = 311 | n = 6 | SA, n = 2; TA, n = 4 | Second trimester, n = 10 | TA, n = 5; SA, n = 1; Live birth, n = 4 (FTND, n = 3; LSCS, n = 1; Prem LCSC, n = 1) |
| Third trimester, n = 15 | Live birth, n = 15 (FT, n = 12; Prem, n = 3) Maternal outcome: toxic death, n = 1; refractory disease, n = 1 | |||
| APL: outcome following ATRA | ||||
| Shapira et al12 | Up to 85% CA | n = 15 | Normal development | |
| Late first trimester, n = 415 | Normal development | |||
| Siu et al13 | — | — | n = 3 | Transient CM, n = 1 Reversible fetal arrhythmia, n = 2 |
| Culligan et al3 | — | n = 1 | LSCS at 30 wk, n = 1 | |
| — | n = 1 (+idarubicin) | Placental abruption and IUFD, n = 1 | ||
| Consoli et al14 | — | — | n = 1 | SVD, Prem, n = 1 (Maternal outcome: postpartum death due to ATRA syndrome) |
| ALL: overall outcome | ||||
| Cardonick8 (N = 60) | n = 38 | No abnormalities, n = 32 | Second trimester, n = 14 | FP, n = 2; IUFD, n = 1; FD, n = 1; CM (transient), n = 1 |
| CA, n = 2; SA, n = 1; Maternal death, n = 1; IUGR, n = 3 | Third trimester, n = 8 | Normal development, n = 8 | ||
| Drugs used in induction-consolidation therapy | ||||
| Cytarabine (alone or in combination) | n = 48,12 | Limb deformities, n = 4 | ||
| n = 898 (all stages of pregnancy) | Cytopenia, n = 5; IUFD, n = 6; IUGR, n = 12; FD due to sepsis, n = 2 | |||
| n = 5210 | Normal delivery, n = 26; IUFD, n = 6; Prem, n = 6; CA, n = 5 IUGR, n = 3; FP, n = 3 | |||
| Anthracyclines | n = 2916 (all stages of pregnancy) | No myocardial damage | ||
| n = 319 | CA, n = 3 | n = 103/n = 26 | CA, n = 2; CM, n = 3 (1 lethal case) | |
| Daunorubicin10 | n = 34 | Normal, n = 15; IUFD, n = 6; Prem, n = 6; CA, n = 2; IUGR = 3; FP, n = 3 | ||
| Idarubicin9 | n = 3 | Reversible CM, n = 2; lethal CM, n = 1 | ||
| Methotrexate | n = 238 | No abnormalities | n = 19 | No abnormalities |
| >10 mg/wk12 | Increased Misc; low birth weight; pancytopenia | Facial and skeletal abnormalities, aminopterin syndrome |
—, data not available; ATRA, all-trans-retinoic acid; CA, congenital abnormality; CM, cardiomyopathy; FD, fetal death; FP, fetal pancytopenia; FT, full term; FTND, full-term normal delivery; FU, follow up; IUFD, intrauterine fetal death; IUGR, intrauterine growth retardation; LSCS, lower segment Caesarean section; Misc, miscarriage; Prem, premature birth; SA, spontaneous abortion; SB, stillbirth; SVD, spontaneous vaginal delivery; TA, therapeutic abortion; TOP, termination of pregnancy.
First trimester
Developmental effects on the fetus are dependent on the point in gestation at which chemotherapy is given. During the preembryonic stage (fertilization until 17 days after conception) rapid cell division occurs. Damage to the majority of the cells of the conceptus is likely to result in miscarriage, but in the event that the injured cells are replaced, it is possible that there will be no long-term effects. Organogenesis occurs in the embryonic period (2-8 weeks following conception)20 and if end-organ damage (heart, neural tube, and limbs) is induced by chemotherapy during this time, the effects are likely to be irreversible. In the fetal period (8-38 weeks after conception), growth and differentiation of the renal and gastrointestinal tract as well as the cerebral cortex continue and remain susceptible to chemotherapy-induced toxicity.6,8 As a result, chemotherapy administered within the first trimester is associated with the greatest risk of miscarriage, fetal death and congenital malformation, ranging from 10% to 20%.21 Chemotherapy also inhibits trophoblast migration and proliferation, which may contribute to neonatal low birth weight, but these data are limited as they are based on infrequent case reports and a small number of retrospective studies.22 Unfavorable experiences of chemotherapy in the first trimester resulted in the recommendation for therapeutic abortion, and in particular, the association of cytarabine and 6-thioguanine with congenital abnormalities led to the recommendation that both of these drugs should be avoided during this time period. Regimen-induced toxicity during the first trimester is well accepted, but as not all fetuses are adversely affected there may be a genetic predisposition to teratogenesis.23 Although the management of AML has evolved since the period of the early reports, with parallel significant improvements in supportive care, cytarabine remains fundamental to most current regimens.24 The decision to treat AML in the first trimester with a regimen containing an antimetabolite, the most effective therapeutic option, must be accompanied by careful counseling of the mother and a sensitive appreciation that many will choose termination of the pregnancy.
Second and third trimesters
The risk of fetal malformation is generally accepted to reduce as the pregnancy advances. Exposure to chemotherapy after the first trimester results in an increased incidence of intrauterine growth retardation (which is also affected by maternal nutritional status throughout), preterm delivery, and fetal death, but no increase in the incidence of congenital abnormalities25 and in particular no documented rise in childhood malignancy or unfavorable neurological development even though the latter continues throughout gestation.26,27 Treatment during the third trimester generally results in the least complications, however, the exact timing of chemotherapy must be carefully planned so as not to induce pancytopenia immediately prior to delivery. Early delivery may be considered if the leukemia presents sufficiently late in pregnancy. If chemotherapy is considered mandatory, then it is important to anticipate the myelosuppressive effects of chemotherapy on the fetus and plan supportive care following delivery which should be timed if possible to coincide with recovery of the maternal blood count. Furthermore, a prolonged duration between chemotherapy administration and fetal delivery will allow for drug elimination through the placenta which may counteract the difficulty of fetal elimination of toxic metabolites due to immaturity of the fetal liver and kidneys.
In summary, following the diagnosis of AML in the first trimester, the patient should be counseled and advised to consider termination of pregnancy. In the second or third trimester, induction chemotherapy with daunorubicin and cytarabine can be introduced, with regular surveillance for the development of congenital abnormalities and monitoring of fetal cardiac function.
APL
The characteristic coagulopathy associated with APL further complicates management in pregnancy, labor, and delivery. Standard management in addition to blood and coagulation support for disseminated intravascular coagulation, includes ATRA and the anthracycline idarubicin, but both of these drugs are problematic in pregnancy.28 ATRA remains pivotal to APL treatment, but if given between 3 and 5 weeks of gestation is associated with a high incidence of fetal malformation, in particular skeletal defects and abnormalities of the neural tube, thymus, heart, and kidneys (Table 1). The European Leukaemia Net recommends avoidance of ATRA in the first trimester, and women should be counseled to consider termination. Leukapheresis is not recommended for APL patients presenting with elevated white blood cell count as this may exacerbate the coagulopathy and increase morbidity.29 Should termination of the pregnancy be unacceptable, treatment with an anthracycline should be started and ATRA introduced in the second trimester. Daunorubicin is the anthracycline of choice, not only because there is more experience of the use of daunorubicin in pregnancy, but also because it may also induce less fetal toxicity than idarubicin, a derivative of daunorubicin. Idarubicin has greater lipophilic properties, a long half-life, is associated with increased placental transfer, and shows higher affinity for deoxyribonucleic acid.30,-32 Chemotherapy alone, however, increases the risk of hemorrhage due to the release of procoagulants and plasminogen activators from malignant cells. Arsenic trioxide which has been used for relapsed APL patients and more recently explored as first-line therapy cannot be recommended at any stage of pregnancy as it is highly embryotoxic.3,33 Similarly, gemtuzumab ozogamicin, which can be highly effective in APL, is not justifiable for use in pregnancy.3
Treatment after the beginning of the second trimester results in a more successful outcome.14,15,34 Chemotherapy does not appear to cause congenital abnormalities,3 but undoubtedly increases the risk of abortion, prematurity, low birth weight, neonatal neutropenia, and sepsis.29 Potentially, ATRA could be given alone with the addition of the anthracycline after delivery. This has resulted in remission rates equivalent to combination ATRA and chemotherapy. However, using ATRA as a single agent increases the risk of ATRA syndrome (APL differentiation syndrome) and possible ATRA resistance.35 This should be carefully monitored and molecular assessment of response can be used to indicate the need to introduce chemotherapy.3,36 Alternatively, ATRA and an anthracycline can be administered and indeed combination therapy is recommended for high-risk patients with hyperleukocytosis and where reverse transcription-polymerase chain reaction (RT-PCR) monitoring for the PML-RARA fusion gene is not practical. As ATRA therapy in pregnancy has been associated with fetal cardiac toxicity including reversible arrhythmias, the importance of cardiac monitoring should be highlighted.3,13,37
In consultation with obstetric colleagues, elective delivery can be scheduled according to fetal maturity and a gestational age of 32 weeks or more is usually acceptable. Antenatal corticosteroids before preterm delivery are recommended to alleviate the complications of respiratory distress syndrome. If possible, a normal delivery is preferred to Caesarean section, in order to reduce the risk of hemorrhage. Following delivery, fetal cardiac monitoring should be recommended.
Acute lymphoblastic leukemia
The incidence of acute lymphoblastic leukemia (ALL) is greater in childhood and adolescence and subsequently there are fewer reports on the outcome in pregnancy. The accepted management of ALL involves an immediate but lengthy regimen of combination chemotherapy for induction, consolidation, and maintenance that includes intrathecal chemotherapy as well as radiotherapy.38 Limited data regarding the treatment of ALL in pregnancy impedes absolute recommendations for management. High-dose methotrexate plays an important part in most protocols, but this agent is recognized to cause aminopterin syndrome (cranial dystosis, ear deformities, and micrognathia).39,-41
From the limited data available it might be argued that chemotherapy could be delivered fairly safely regardless of trimester (Table 1). However, it is important to note that all pregnancies that resulted in TOP without autopsy data were excluded in the largest report, so the incidence of fetal abnormalities may be significantly greater. Bearing this in mind, a more conservative approach may be appropriate; for those presenting in the first trimester termination should be considered, while for those diagnosed later a modified treatment regimen can be proposed without methotrexate until the third trimester, when patients can be treated in the same way as their nonpregnant counterparts. The period of pregnancy can be viewed in 2 partitions: before and after 20 weeks of gestation. Before 20 weeks gestation, termination should be considered, and then conventional therapy instituted.5,12 Following 20 weeks’ gestation, bridging chemotherapy without methotrexate until the third trimester can be instituted. A brief period of treatment with prednisolone alone for 1 to 2 weeks may allow the patient to enter the period of gestation past 20 weeks in order to then receive more intensive chemotherapy. A similar approach with prednisolone alone can also be recommended for patients presenting close to 32 weeks of pregnancy.12
The outcome of ALL is stratified according to a number of risk factors and patients with a good prognosis could have less-intensive chemotherapeutic approaches, and equally those with more aggressive features will require intervention according to the pace of the underlying disease. Regimens in pregnancy have included cytarabine, cyclophosphamide, l-asparaginase anthracyclines, vincristine, and steroids.42 As with AML, elective delivery after 32 weeks should be planned while aiming to avoid pancytopenia peridelivery.
Supportive care
A number of additional supportive agents are usually used to ensure that the chemotherapy is well tolerated, including antiemetics, commonly ondansetron43 and metoclopramide,44 as these appear safe in pregnancy. During the neutropenic period, patients may require treatment of sepsis, and the choice of antimicrobials is dependent on local institutional policies usually incorporating Gram-positive and -negative cover. Teicoplanin (Food and Drug Administration [FDA] pregnancy category B3)45 is associated with increased rate of stillbirth in animal studies and should only be used where the benefit outweighs the risk.46 Data on pregnancy outcomes following treatment with vancomycin indicates no malformative or feto/neonatal toxicity, however, as vancomycin was administered only in the second and third trimesters, it is not precisely known whether it causes fetal harm. Vancomycin blood levels are mandatory in order to minimize the risk of fetal toxicity and pregnant patients may require greatly increased doses of vancomycin in order to achieve therapeutic serum concentrations.47 Penicillins, cephalosporins, aminoglycosides, and metronidazole seem to be safe, although experience is limited. Sulphonamides and tetracyclines should be avoided where possible.48,49 Ordinarily ciprofloxacin is a commonly used agent for the prevention of neutropenic fever in nonpregnant patients. Of 549 cases reported involving fluoroquinolone exposure, congenital malformations were reported in 4.8%, however, this was not higher than the background rate. Ciprofloxacin has been assigned to pregnancy category C by the FDA.45 Animal studies failed to reveal embryotoxicity or teratogenicity, although maternal toxicity in some animal studies resulted in increased incidence of abortion and ciprofloxacin has been shown to distribute into amniotic fluid. As safer alternatives are available, ciprofloxacin is generally considerate as contraindicated during pregnancy, especially during the first trimester, and ciprofloxacin is only used when the clinical benefit outweighs risk.50 To aid neutrophil recovery, granulocyte colony-stimulating factor can be used without adverse effects.51
Antifungal therapy
Early in their clinical use, it became apparent that azole antifungals could be teratogenic, possibly due to aberrant sterol metabolism (Table 252 ).53,54 Exposure up to 23 weeks’ gestation can cause severe neonatal dysmorphic features including craniosynostosis, cleft palate, humeral-radial fusion, bowed tibia and femur, hypoplasia of the nasal bones, and short thumbs and toes in women receiving fluconazole at doses of ≥400 mg daily during the first trimester of pregnancy. A number of studies have reported a lack of congenital defects among infants delivered by women exposed to short courses of lower doses of fluconazole, including when given during the first trimester. There are few data on safety of fluconazole after the first trimester in humans, but the reports that have been published suggest that exposure later in pregnancy may be safe, however, fluconazole use was limited to low-dose, short courses.55 Recently, an observational study of itraconazole during pregnancy did not find a greater incidence of congenital abnormalities but did note an increased risk of spontaneous abortion.56 Itraconazole and posaconazole have also been found to cause similar craniofacial and skeletal abnormalities in animal models, but often require high doses to cause such abnormalities. According to the FDA pregnancy categories,45,52 itraconazole and fluconazole belong to category C. Voriconazole, which has been associated with congenital malformations at subtherapeutic doses, is currently listed as a class D agent.57 Animal studies have not shown AmBisome to exhibit teratogenic potential but the safety of AmBisome in pregnant women has not been fully established. Although systemic fungal infections have been uneventfully treated in pregnant women with conventional amphotericin B, the number of cases reported are few and cannot be transposed to the safety of AmBisome in pregnancy, although uneventful courses have been reported.58 The few studies available on amphotericin pharmacokinetics in pregnant women indicate that it will cross the placenta and achieve therapeutic concentrations in the fetal circulation. This may contribute to transient neonatal renal dysfunction if given in the third trimester.59 Caspofungin (assigned to pregnancy category C by the FDA) has shown developmental toxicity (skeletal abnormalities and periimplantation losses) in animal reproductive studies at therapeutic doses and is recommended only if the potential benefit justifies the risk to the fetus.60 Effectively, nonliposomal amphotericin has the most robust evidence base for safe use in pregnancy of all the current available agents, but this needs to be rationalized against the toxicity of the preparation against the newer formulations. Mouth care for nonpregnant neutropenic patients incorporates nystatin oral suspension and although treatment with oral nystatin during pregnancy appears to present little teratogenic risk to the fetus, there are reports of a possible association with hypospadias, leading to a recommendation that the need for nystatin therapy in pregnancy needs to be balanced against the risk to the fetus.61 The specific antifungal therapy for treatment and prophylaxis will be directed by the institutional guidelines of the individual center with careful consideration of the toxicity profile of each agent.
Recommendations for antifungal use in pregnancy
| Antifungal . | FDA pregnancy category* . | Placental transfer . | Animal data . | Clinical data . |
|---|---|---|---|---|
| Amphotericin (AmBisome) | B | Yes | No teratogenic harmIn high dose, SA | Much successful data; transient neonatal renal dysfunction if given in the third trimester (safety unclear) |
| Caspofungin | C | Yes | Skeletal abnormalities | Avoid in pregnancy in the first trimester. |
| Fluconazole | D (C if single 150 mg dose) | LMW makes passage likely | Skeletal and cardiac malformations for long-term high-dose use | Skeletal and cardiac malformations for long-term high-dose use; avoid in pregnancy |
| Itraconazole | C | LMW makes passage likely | Major skeletal defects, encephaloceles, macroglossia | Risk of structural anomalies (skeletal, cardiac, renal) is low; increase in SA. Avoid in pregnancy unless benefit outweighs risk |
| Posaconazole | C | LMW makes passage likely | No data. Avoid in pregnancy unless benefit outweighs risk | |
| Voriconazole | D | LMW makes passage likely | Strongly associated with teratogenicity: cleft palate, hydronephrosis, bone abnormalities | Avoid in pregnancy unless benefit outweighs risk |
| Antifungal . | FDA pregnancy category* . | Placental transfer . | Animal data . | Clinical data . |
|---|---|---|---|---|
| Amphotericin (AmBisome) | B | Yes | No teratogenic harmIn high dose, SA | Much successful data; transient neonatal renal dysfunction if given in the third trimester (safety unclear) |
| Caspofungin | C | Yes | Skeletal abnormalities | Avoid in pregnancy in the first trimester. |
| Fluconazole | D (C if single 150 mg dose) | LMW makes passage likely | Skeletal and cardiac malformations for long-term high-dose use | Skeletal and cardiac malformations for long-term high-dose use; avoid in pregnancy |
| Itraconazole | C | LMW makes passage likely | Major skeletal defects, encephaloceles, macroglossia | Risk of structural anomalies (skeletal, cardiac, renal) is low; increase in SA. Avoid in pregnancy unless benefit outweighs risk |
| Posaconazole | C | LMW makes passage likely | No data. Avoid in pregnancy unless benefit outweighs risk | |
| Voriconazole | D | LMW makes passage likely | Strongly associated with teratogenicity: cleft palate, hydronephrosis, bone abnormalities | Avoid in pregnancy unless benefit outweighs risk |
LMW, low molecular weight. Other abbreviations are explained in Table 1.
FDA pregnancy categories: (Category A) Adequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters). (Category B) Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. (Category C) Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. (Category D) There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.52
Chronic leukemias
Chronic myeloid leukemia
Chronic myeloid leukemia (CML) accounts for 15% of adult leukemias, but only a small proportion of patients are diagnosed during childbearing age as the median age at diagnosis is in the sixth decade. CML occurs in up to 10% of pregnancy-associated leukemias, with an annual incidence of 1 per 100 000 pregnancies.62 The diagnosis of CML during pregnancy may be made more complicated as the physiological changes, including those in hematological parameters which accompany pregnancy, may mask the symptoms. Previously, there was a suggestion of an increase in miscarriage rates, low birth weight, and premature babies in CML mothers but this is no longer apparent in more recent reports. Reassuringly, the course of the disease does not appear to be affected by pregnancy.63 The prothrombotic potential of a normal pregnancy is well recognized as a result of a physiological increase in hemostatic factors and prothrombotic proteins in addition to the physical obstruction of venous blood flow. As a result, thrombosis continues to be the most common cause of maternal morbidity and this may be compounded in the myeloproliferative diseases where there is an associated elevation in the platelet count.
Due to the excellent clinical outcome with oral-targeted therapy, the expectation of a relatively normal lifestyle inclusive of parenting children is increasing. However, treatment of the great majority of patients is lifelong so in contrast to acute leukemia, patients with CML may not only present in pregnancy but also wish to become pregnant while on active treatment. Since the introduction of imatinib, more potent second-generation tyrosine kinase inhibitors (2G-TKIs) are now available for imatinib-resistant patients as well as being positioned for first-line use. The TKIs share a number of class effects due to their inhibition of BCR-ABL1 and also have a number of off-target effects as a result of the inhibition c-kit, the platelet-derived growth factor receptors, arg and c-fms.64 Furthermore, dasatinib, 1 of the 2G-TKIs, also inhibits Src and related proteins. A number of these proteins are relevant to gonadal development, embryonic implantation, and fetal maturation.65
Therapeutic approaches for CML diagnosed in pregnancy have included supportive care in the form of leukapheresis, chemotherapy (hydroxycarbamide [HC]), interferon-α (IFN-α), and imatinib.64 There are numerous case reports describing the use of leukapheresis and platelet pheresis in pregnancy in CML as a means of controlling the blood counts in order to avoid potentially teratogenic drugs (Table 365 ),66 but unfortunately leukapheresis is not universally available.67 Individuals are occasionally unable to tolerate the required frequency of leukapheresis and venous access can be problematic, however, requirements of leukapheresis notably reduce in the third trimester. IFN-α does not inhibit DNA synthesis and is considered safe in pregnancy subsequent to animal studies and numerous reports in the literature.68,69 IFN-α has a high molecular weight of 19 kDa and should not cross the placenta.
Leukapheresis in pregnancy: outcome of patients at Hammersmith Hospital
| CML individual pregnancy managed by leukapheresis . | WCC at diagnosis, ×109/L . | Leukapheresis regimen . | Other treatment . | Duration of pregnancy,* wk . | Live infant . |
|---|---|---|---|---|---|
| 1-2 weekly until 26-30 wk, n = 7 | None, n = 10 | Live infant, n = 11 | |||
| N = 1265 | 25-240 (90) | 1-4 × wk, n = 1 | HU from 30 wk, n = 1 | 35-40 (39) | Live infant, meningomyelocele and talipes,† n = 1 |
| 2-3 × wk, n = 4 | Aspirin and LMWH, n = 1 | ||||
| CML individual pregnancy managed by leukapheresis . | WCC at diagnosis, ×109/L . | Leukapheresis regimen . | Other treatment . | Duration of pregnancy,* wk . | Live infant . |
|---|---|---|---|---|---|
| 1-2 weekly until 26-30 wk, n = 7 | None, n = 10 | Live infant, n = 11 | |||
| N = 1265 | 25-240 (90) | 1-4 × wk, n = 1 | HU from 30 wk, n = 1 | 35-40 (39) | Live infant, meningomyelocele and talipes,† n = 1 |
| 2-3 × wk, n = 4 | Aspirin and LMWH, n = 1 | ||||
HU, hydroxyurea; LMWH, low-molecular-weight heparin; WCC, white cell count.
Data not known in 1 case.
Treatment with leukapheresis only.
A number of distinctive congenital abnormalities have been described after exposure to imatinib in early pregnancy, including skeletal malformations (premature closure of skull sutures, craniosynostosis, absent hemivertebrae, shoulder anomaly, and scoliosis), renal (duplex kidney, renal agenesis), respiratory (hypoplastic lungs), and gastrointestinal (exomphalos, omphalocele) abnormalities (Table 466,70,,,,,,,,-79 ).70,-72 In particular, the incidence of exomphalos in these cases is roughly 100-fold greater than expected and a cause for significant concern. In animal studies, imatinib was found to be teratogenic in mice but not in rabbits and these abnormalities are postulated to be as a result of PDGFRA inhibition.80 The duration of imatinib therapy was not known in all of the affected cases described and as such it is not possible to predict an exact correlation between cumulative TKI dosage and the development of congenital abnormalities. In cases where conception has occurred on imatinib, imatinib should be stopped and close monitoring of fetal development should be recommended, including a nuchal scan for fetal anomaly, regardless of maternal age. The parents should be informed of the known fetal potential risks and a judgment should be made according to the risk of transformation for the mother in light of the existing disease response to therapy as effective treatment needs to be interrupted. Despite this recommendation, as imatinib does not cross the placenta, some physicians have chosen to treat CML patients in pregnancy from the second trimester, and there are cases where imatinib has not been discontinued upon conception.73 There is significantly less experience with 2G-TKIs (bosutinib, dasatinib, nilotinib, and ponatinib) in pregnancy.75 Dasatinib, a dual BCR-ABL/src kinase inhibitor crosses the placenta and leads to considerable levels in fetal plasma.81 In the first trimester, dasatinib has been reported to cause fetal hydrops and severe fetal bicytopenia,76 but normal pregnancies have also been reported.74,77,-79
CML in pregnancy outcome following therapy
| CML . | First trimester . | Outcome . | Second/third trimester . | Outcome . |
|---|---|---|---|---|
| HC | ||||
| N = 566 ; continued throughout pregnancy | Normal infant, n = 4; eclampsia at 26 wk; stillbirth, n = 1 | |||
| Imatinib | ||||
| N = 180 (outcome, n = 125)70 | 70% | SA, n = 4 | n = 18 (26%); imatinib throughout pregnancy | SA, n = 18; TOP, n = 35 (CA, n = 3); total CA, n = 12; normal infants, n = 63 |
| N = 1071 | SA, n = 2 | |||
| N = 21772 | Pregnancy to term, n = 171 (n = 109 FU; UNK = 62); SA, n = 24; CA, n = 9; LBW, n = 2; IUFD, n = 1 | |||
| Imatinib continued throughout pregnancy, n = 273 | Pregnancy to term; LBW, n = 2 | |||
| 2G-TKI | ||||
| Dasatinib, N = 4 | n = 176 | Fetal hydrops and cytopenia | ||
| n = 1 (5/40 wk)77 | Normal pregnancy, induction of labor | |||
| n = 1(4/40 wk)78 | Normal pregnancy, LSCS at 33 wk | |||
| n = 1 (6/40 wk)79 | Normal pregnancy, SVD at 37/40 wk | |||
| Dasatinib,74 N = 8 | TOP, n = 3 SA, n = 2 (8/40 and 9/40) Deliveries, n = 3; normal infant, n = 1 | |||
| LSCS at 7 mo, “small for dates” | ||||
| Normal pregnancy at 21 wk, outcome unknown | ||||
| Nilotinib,75 N = 1 | 8/40 wk | Normal pregnancy LSCS at 33 wk | ||
| CML . | First trimester . | Outcome . | Second/third trimester . | Outcome . |
|---|---|---|---|---|
| HC | ||||
| N = 566 ; continued throughout pregnancy | Normal infant, n = 4; eclampsia at 26 wk; stillbirth, n = 1 | |||
| Imatinib | ||||
| N = 180 (outcome, n = 125)70 | 70% | SA, n = 4 | n = 18 (26%); imatinib throughout pregnancy | SA, n = 18; TOP, n = 35 (CA, n = 3); total CA, n = 12; normal infants, n = 63 |
| N = 1071 | SA, n = 2 | |||
| N = 21772 | Pregnancy to term, n = 171 (n = 109 FU; UNK = 62); SA, n = 24; CA, n = 9; LBW, n = 2; IUFD, n = 1 | |||
| Imatinib continued throughout pregnancy, n = 273 | Pregnancy to term; LBW, n = 2 | |||
| 2G-TKI | ||||
| Dasatinib, N = 4 | n = 176 | Fetal hydrops and cytopenia | ||
| n = 1 (5/40 wk)77 | Normal pregnancy, induction of labor | |||
| n = 1(4/40 wk)78 | Normal pregnancy, LSCS at 33 wk | |||
| n = 1 (6/40 wk)79 | Normal pregnancy, SVD at 37/40 wk | |||
| Dasatinib,74 N = 8 | TOP, n = 3 SA, n = 2 (8/40 and 9/40) Deliveries, n = 3; normal infant, n = 1 | |||
| LSCS at 7 mo, “small for dates” | ||||
| Normal pregnancy at 21 wk, outcome unknown | ||||
| Nilotinib,75 N = 1 | 8/40 wk | Normal pregnancy LSCS at 33 wk | ||
LBW, low birth weight; UNK, unknown outcome. Other abbreviations are explained in Table 1.
HC is a cytotoxic agent that inhibits RNA synthesis and is commonly used for cytoreduction in newly diagnosed CML, prior to therapy with TKI. HC does not alter the natural history of CML, and is well recognized to cause embryotoxicity in many animal species,69 including craniofacial and spinal defects, fetal growth restriction, and intrauterine death.82 However, HC appears to be less damaging than might be anticipated in human pregnancies, with a number of unremarkable outcomes following HC exposure in early pregnancy. In a series of 5 case reports of CML where HC was continued throughout gestation, only 1 pregnancy resulted in a stillbirth of a morphologically normal infant at 26 weeks as a result of eclampsia, with the other pregnancies being unaffected.66
In summary, for a patient presenting with CML in chronic phase in the first trimester, treatment is probably unnecessary if the white cell count remains below 100 × 109/L and the platelet count is <500 × 109/L. Leukapheresis is recommended to maintain a threshold below these levels. The frequency of leukapheresis will naturally be tailored to the individual and vary according to the gestation, but in general can be performed as often as alternate days to 1 to 2 weekly. Low-molecular-weight heparin as well as aspirin can be used once platelets exceed 1000 × 109/L. For women who are leukapheresis intolerant or for whom it proves ineffective, IFN-α is an option after the second trimester. HC is best avoided unless there is no alternative. For women who present in accelerated phase, the pace of the disease needs to be carefully considered and advice offered accordingly. In cases of CML in blast crisis in early pregnancy, which remains an aggressive phase of the disease with a poor prognosis regardless of TKI therapy, the recommendation for management is similar to that for those presenting with acute leukemia in pregnancy.
Outcome data on TKIs in pregnancy continues to be collected83 and pharmacovigilance remains important to increase our experience in these cases. It is clear that exposure to TKIs during pregnancy may result in an increased risk of serious fetal abnormalities or spontaneous abortion. Women of child-bearing potential should continue to use adequate contraception while taking TKI therapy.
Management of pregnancy while on treatment
With first- and/or second-generation TKI, most patients will achieve deep and durable responses, consistent with a normal life expectancy. As a consequence, many women are seeking advice regarding the feasibility and safety of becoming pregnant while on treatment. Because observed congenital abnormalities have occurred with the use of TKI in the first trimester, patients should be advised to discontinue treatment before conception. Confidence in withdrawing imatinib has been gained from “stopping imatinib” studies which show that roughly 40% of patients continue to maintain a deep response with undetectable BCR-ABL1 transcripts when imatinib has been discontinued after the achievement of a complete molecular response (CMR) for a period of 2 years.84,85 Furthermore, the rate of loss of CMR was diminished if the period of CMR had been for >5 years. In those patients that relapsed, 26 of 42 patients regained CMR at the time of last follow up. Unfortunately, the proportion of patients achieving these prolonged and deep responses on imatinib is <10% and although early data from the use of 2G-TKI as first-line therapy suggest that this percentage may be higher in future, there is limited information on the durability of CMR after stopping these drugs. Most women wishing to become pregnant will either not be in such deep remissions or will not have sustained these responses for several years.
Many, however, will have achieved a 3-log reduction in tumor load defined as a major molecular response (MMR). Reassuringly, adequate responses to restarting imatinib after discontinuation in pregnancy have been seen in patients in MMR prior to stopping the drug (Table 571 ).86 Some suboptimal responders demonstrated the same response upon drug re-introduction, but of concern, a number of patients failed imatinib therapy and required a change to a 2G-TKI. The unsatisfactory results after the reintroduction of imatinib are related to either an inadequate response prior to conception or to the fact that these were poor-risk patients. It is possible that the use of nilotinib or dasatinib in suboptimal responders in order to obtain MMR prior to therapy discontinuation may reduce the risk of treatment failure after the re-introduction of therapy.
Outcome of imatinib discontinuation for conception
| . | Time on IM prior to discontinuation, mo . | Response at time of discontinuation . | Time off IM, mo . | Outcome on imatinib retreatment . | |
|---|---|---|---|---|---|
| N = 1071 | |||||
| 1-52 (8) | Not in CHR, n = 1 | 1-21 (7) | Minor CyR, n = 2 | ||
| (CP, n = 9; | Minor CyR = 2 | PCyR, n =3 | CHR, n = 9 | ||
| AP, n = 1) | No CyR, n = 3 | CCyR, n = 3 | |||
| PCyR, n = 3 | UNK, n = 1 | ||||
| CCyR, n = 1 | Transformation to BP, n = 1 | ||||
| N = 786 | |||||
| Sokal score | 1-50 (14) | PCyR, n = 1 | 6-23 (9) | Previous response maintained, n = 3 | |
| High, n = 1 | CCyR, n = 3 | (MMR, n = 2;CCyR, n = 1) | |||
| Low, n = 6 | MMR, n = 3 | Improvement, n = 1 | |||
| (CMR, n = 1) | |||||
| Loss of response, n = 3 | |||||
| (change to 2G-TKI, n = 2*) | |||||
| . | Time on IM prior to discontinuation, mo . | Response at time of discontinuation . | Time off IM, mo . | Outcome on imatinib retreatment . | |
|---|---|---|---|---|---|
| N = 1071 | |||||
| 1-52 (8) | Not in CHR, n = 1 | 1-21 (7) | Minor CyR, n = 2 | ||
| (CP, n = 9; | Minor CyR = 2 | PCyR, n =3 | CHR, n = 9 | ||
| AP, n = 1) | No CyR, n = 3 | CCyR, n = 3 | |||
| PCyR, n = 3 | UNK, n = 1 | ||||
| CCyR, n = 1 | Transformation to BP, n = 1 | ||||
| N = 786 | |||||
| Sokal score | 1-50 (14) | PCyR, n = 1 | 6-23 (9) | Previous response maintained, n = 3 | |
| High, n = 1 | CCyR, n = 3 | (MMR, n = 2;CCyR, n = 1) | |||
| Low, n = 6 | MMR, n = 3 | Improvement, n = 1 | |||
| (CMR, n = 1) | |||||
| Loss of response, n = 3 | |||||
| (change to 2G-TKI, n = 2*) | |||||
AP, accelerated phase; BP, blast phase; CCyR, complete cytogenetic response; CHR, complete hematological response; CP, chronic phase; CyR, cytogenetic response; IM, imatinib; MMR, major molecular response; PCyR, partial cytogenetic response. Other abbreviations are explained in Table 4.
One patient lost to follow up.
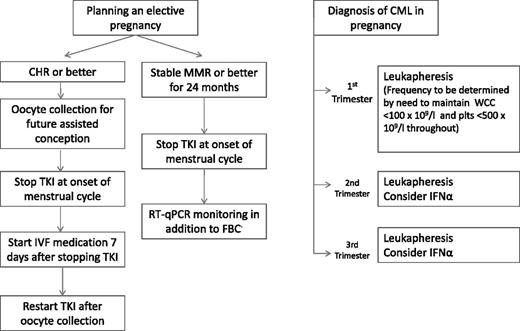
Based on the current evidence, it would seem reasonable to recommend that women with CML who wish to become pregnant should be advised to wait until they have achieved MMR and sustained this for at least 2 years. Imatinib can be discontinued shortly before ovulation, perhaps at the time of menstruation. The duration of time off drug before conception should be limited as this period will be added to the duration of the pregnancy as the total time off treatment; 6 months might be acceptable although this could be extended if serial RT quantitative PCR (RT-qPCR) analyses for BCR-ABL1 transcripts do not show a rise from baseline. For women whose responses are less deep and/or of shorter duration, consideration might be given to the use of in vitro fertility techniques to achieve either rapid pregnancy or embryo storage for reimplantation after a further period of treatment (Figure 1).
Suggested algorithm for management of pregnancy in CML. The RT-qPCR will usually rise off treatment but the increase is not necessarily a trigger for intervention. The first measurement should be performed 2 to 3 months after stopping treatment but thereafter the frequency of monitoring will be guided by the rate of the initial increase. FBC, full blood count; IVF, in vitro fertilization; plts, platelets.
Suggested algorithm for management of pregnancy in CML. The RT-qPCR will usually rise off treatment but the increase is not necessarily a trigger for intervention. The first measurement should be performed 2 to 3 months after stopping treatment but thereafter the frequency of monitoring will be guided by the rate of the initial increase. FBC, full blood count; IVF, in vitro fertilization; plts, platelets.
Chronic lymphocytic leukemia
The median age at diagnosis of chronic lymphocytic leukemia (CLL) makes the diagnosis of pregnancy in this condition unlikely. The often indolent nature of CLL allows for later interventions, if any therapy at all is required, but there may be a risk of leukostasis, placental insufficiency and subsequent low fetal birth weight, increased fetal prematurity, and increased mortality if the leukemia is left unattended for the duration of pregnancy. Less than 10 cases have been reported in the literature since 1996.87,88 Therapeutic options include leukapheresis,87 chlorambucil, and more recently rituximab, a chimeric anti-CD20 monoclonal B-cell–depleting antibody. Although chlorambucil is embryotoxic (neural tube defects, skeletal and renal abnormalities), successful outcomes in pregnancy have been reported despite chlorambucil exposure in the first trimester. The assessment of rituximab exposure during pregnancy has been confounded by concomitant chemotherapy use, and although few congenital malformations or neonatal infections have ensued, women should continue to avoid pregnancy for ≥12 months after rituximab exposure until more definitive data are available.89
Hairy cell leukemia
Hairy cell leukemia accounts for 2% to 3% of adult leukemias, but on account of male predominance and the late median age at diagnosis, it is uncommon in pregnancy. Fewer than 10 cases have been reported.90,91 Hairy cell leukemia can be complicated by significant splenomegaly, and treatment options have traditionally included splenectomy and single-agent 2-chlorodeoxyadenosine (cladrabine). If possible, it is preferable to defer treatment until after delivery. Patients have been variably treated with IFN, rituximab, and with splenectomy, including laparascopic splenectomy, in the second trimester.
Breastfeeding
Chemotherapeutic agents differ in their concentration found in breast milk, and although definitive neonatal toxicity during lactation has not been precisely delineated, it would be advisable to avoid breastfeeding for a period of 2 weeks or more after the administration of chemotherapy.
Specific to some of the agents mentioned above:
ATRA should be avoided due to the potential for ATRA-induced cardiac toxicity and arrhythmias. Similar recommendations apply to arsenic trioxide.
IFN-α is probably excreted in breast milk and patients should be advised not to breastfeed.92
HC is excreted in breast milk and should be avoided during lactation.
From information obtained from animal models, it is clear that imatinib, its metabolites, and 2G-TKIs are actively excreted in breast milk, which is also the case in human breast milk.93 Although a number of reports exist that show no harm to the developing infant during the last reported follow up, the general recommendation is to avoid TKIs during lactation.94
Ciprofloxacin is excreted into human milk and there have been isolated reports of pseudomembranous colitis, cartilage erosion, and arthropathies in nursing infants. Although ciprofloxacin is considered compatible with breastfeeding by the American Academy of Pediatrics, the decision to cease nursing or discontinue administration of ciprofloxacin should be rationalized according to the importance of the drug to the mother.
There are few data on the use of azole antifungals during breastfeeding. Both fluconazole and itraconazole enter breast milk. Following a single dose of fluconazole, >80% of the plasma concentration is detected in breast milk. Dependent on the dose, this may be less than concentrations for prescribed doses for neonates. Itraconazole has been found to enter breast milk at low concentrations but may accumulate over time. The American Academy of Pediatrics considers fluconazole to be compatible with breastfeeding but women should not consider breastfeeding while receiving itraconazole, posaconazole, or voriconazole, for which there are no data. It is unknown whether AmBisome is excreted in human breast milk. There is a similar lack of information for caspofungin, and although there is no published experience, as Caspofungin is indicated for use in infants over 3 months of age and it is poorly absorbed orally, it is not likely to reach the bloodstream of the infant.
Late fetal effects
The impact of maternal intervention for leukemia on neurological development, cardiac impairment, fertility, and risk of malignancy to the offspring has been of concern and long-term follow up has remained challenging. An understanding of the long-term effects of future childhood malignancy after in utero chemotherapy exposure is even more limited due to the relatively low incidence of childhood cancer.4,95 Reassuringly, the influence of maternal chemotherapy on the long-term neonatal outcome appears to be minimal (Table 616,26,27,95,96 ).96
Long-term fetal outcome following chemotherapy exposure
| . | Follow up . | Outcome . | |
|---|---|---|---|
| Development . | Incidence of cancer . | ||
| Hematologic malignancy, N = 84; (AL, n = 29)26 | Age: 6-29 y (median, 18.7 y) | No congenital, neurological, or psychological abnormalities | No increased risk |
| First-trimester exposure, n = 38 | Normal reproductive development in 12 second-generation children | ||
| In utero exposure to chemotherapeutic agents, N = 111; (AL, n = 17)27 | Assessed age: 4-22 y | Normal neurodevelopment | — |
| Exposure to anthracyclines in utero, N = 81; (AL, n = 29)16 | Age: 9.3-29.5 y (mean 17.1 y) | No cardiac toxicity | — |
| Exposure to chemotherapy in utero post first trimester,95 N = 157 | Mean neonatal follow up: 3 y postpartum | No increase in congenital anomalies, preterm delivery, or growth restriction | — |
| N = 7096 | Median follow-up period of 22.3 mo (range, 16.8-211.6 mo) | No association with increased neurologic, cardiac, or auditory morbidity; no difference in overall health and growth compared with the general population | — |
| . | Follow up . | Outcome . | |
|---|---|---|---|
| Development . | Incidence of cancer . | ||
| Hematologic malignancy, N = 84; (AL, n = 29)26 | Age: 6-29 y (median, 18.7 y) | No congenital, neurological, or psychological abnormalities | No increased risk |
| First-trimester exposure, n = 38 | Normal reproductive development in 12 second-generation children | ||
| In utero exposure to chemotherapeutic agents, N = 111; (AL, n = 17)27 | Assessed age: 4-22 y | Normal neurodevelopment | — |
| Exposure to anthracyclines in utero, N = 81; (AL, n = 29)16 | Age: 9.3-29.5 y (mean 17.1 y) | No cardiac toxicity | — |
| Exposure to chemotherapy in utero post first trimester,95 N = 157 | Mean neonatal follow up: 3 y postpartum | No increase in congenital anomalies, preterm delivery, or growth restriction | — |
| N = 7096 | Median follow-up period of 22.3 mo (range, 16.8-211.6 mo) | No association with increased neurologic, cardiac, or auditory morbidity; no difference in overall health and growth compared with the general population | — |
AL, acute leukemia. Other abbreviations are explained in Table 1.
Conclusion
The approach to the management of leukemia in pregnancy remains a substantial challenge and involves consideration of the nature of the disease, the phase of the leukemia, the necessity for intervention, and a careful evaluation of maternal and fetal risk. For the more aggressive leukemias presenting in the first trimester, termination of pregnancy is often advisable. For leukemias with a more indolent course and for those diagnosed later in pregnancy, treatment can be adjusted to provide the most favorable outcome. National registries are to be strongly encouraged to obtain experience in the outcomes and management of this relatively rare condition in order to allow an increase in the available expertise and accuracy of the recommendations available.
Authorship
Contribution: D.M. and J.F.A. performed the literature review, discussed their opinion, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dragana Milojkovic, Catherine Lewis Centre, 2nd Fl, Hammersmith Hospital Site, Du Cane Rd, London W12 0HS, United Kingdom; e-mail: d.milojkovic@imperial.ac.uk.