Abstract
Patients with hemophilia, who have a lifelong hypocoagulability, seem to have a lower cardiovascular mortality than the general population. Nevertheless, the prevalence of cardiovascular risk factors in patients with hemophilia is as prevalent as in the general population, and hypertension is even more common. Furthermore, hemophiliacs have the same degree of atherosclerosis as the general population. The reduced cardiovascular mortality may be explained by reduced thrombus formation resulting from hypocoagulability. On the other hand, hemophilia, which is associated with reduced thrombin generation, may also increase atherosclerotic plaque stability, as has been shown in mice. Because treatment of these events is extremely challenging in patients with increased bleeding tendency, detection and aggressive treatment of risk factors is mandatory.
Introduction
The concept of risk factors in cardiovascular disease (CVD) has been well established. Smoking, hypertension, obesity, hypercholesterolemia, diabetes mellitus, and a positive family history for CVD are all associated with an increased risk of morbidity and mortality because of CVD. A prothrombotic state also contributes to the development of CVD.1 Increased levels of fibrinogen, von Willebrand factor (VWF), and factor VIII have all been linked to arterial thrombosis.2,3 VWF is essential for platelet adhesion and aggregation. Furthermore, VWF acts as the carrier protein for coagulation factor VIII. Factor VIII contributes to the formation of a fibrin-rich clot and also has a role in the formation of occluding thrombi in stenotic vessels. Several observational studies have suggested that patients with hemophilia A, who have a congenital deficiency of clotting factor VIII, may have a lower mortality because of arterial thrombosis compared with the general population.4,5 This protection may be due to hypocoagulability, which is associated with decreased thrombin generation and results in inhibition of thrombus formation.
Recently, several studies found that hemophilia A patients, like the general population, have a high prevalence of atherosclerotic plaques.6,7 Furthermore, classical cardiovascular risk factors, such as hypertension, seem even more prevalent in hemophilia patients. Indeed, in clinical practice, an increasing number of hemophilia patients are diagnosed with CVD. The antithrombotic treatment of patients with a lifelong hypocoagulability and consequently a higher bleeding risk is a major challenge in clinical practice.
In this review, we discuss recent developments of cardiovascular risk factors and atherosclerosis in patients with hemophilia.
Hemophilia and CVD
Because both elevated levels of clotting factor VIII and VWF increase the risk of arterial thrombosis,2,3 hemophilia, which is associated with a lifelong hypocoagulable state, may theoretically offer protection against CVD (ie, acute coronary syndrome, stroke, or peripheral vascular disease). A decrease in factor VIII reduces thrombin generation and to a lesser extent platelet function, 2 important mediators in the formation of occluding thrombi in stenotic vessels.
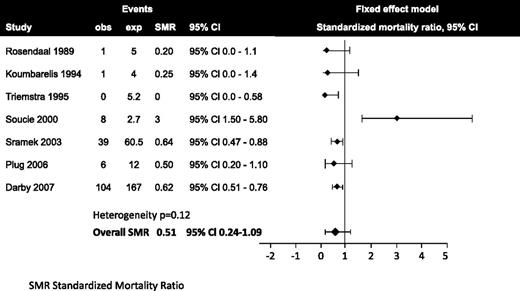
Indeed, several surveys and cohorts of patients with hemophilia showed that cardiovascular mortality was lower compared with the general population. A systematic review summarized the results of 15 longitudinal and cross-sectional studies consisting of 19 242 patients, 14 754 with hemophilia A, 3408 with hemophilia B, 965 carriers of hemophilia A and B, and 115 von Willebrand disease patients.8 Mortality from CVD was nonsignificantly reduced in patients with hemophilia compared with the general population, with a standardized mortality ratio of 0.51 (95% confidence interval [CI] 0.24-1.09) (Figure 1). Six studies consistently found a reduced cardiovascular mortality in patients with hemophilia compared with the general male population. Only the study by Soucie et al9 found a 3-fold increased cardiovascular mortality of hemophilia patients. The reason for the difference is unclear. A more recent study comes from Sweden, where 1431 patients with hemophilia A or B were compared with 7150 controls by using a registry.10 Thirteen percent of the hemophiliacs died from cardiovascular mortality compared with 29% of the controls. Mortality from stroke was not different between the 2 groups.
The influence of hemophilia on nonfatal CVD was investigated in the National Hospital Discharge survey in the United States.11 Among 45- to 64-year-old hemophiliacs, the discharge rate (per 1000) of CVD was 24.1, 50% lower compared with that of US males (48.9/1000). This difference was 30% among patients of 64 years and older (127.3 vs 175.3, respectively). In addition, the incidence of CVD was higher in patients with mild hemophilia (3.4%) than in moderate-severe (0.7%) or severe (0.4%) types of hemophilia (P < .001). In a retrospective single-center analysis from The Netherlands, data from 408 hemophilia patients (204 severe, 204 nonsevere) born before 1971 were compared with the Dutch age-matched general male population.12 The incidence of CVD was 2.5% in the hemophiliacs compared with 4.8% in the general population, which was statistically different. Occurrence of stroke was not different, around 1% in both groups. A third study from a US hemophilia center found that the lifetime risk of CVD for 185 patients with hemophilia was 19.5%.13 Of note, CVD was defined as related to diseases of the heart and blood vessels, which is much broader than only cardiovascular events, and also included valvular heart disease, rhythm disturbances, and congestive cardiomyopathy. However, the rates of cardiovascular events (1.75-fold higher) and of stroke (2.27-fold higher) were higher in hemophiliacs than in the general population. Notably, nearly 40% of the hemophilia patients were older than 55 years, which is higher than in most other studies and can therefore better assess the true prevalence of CVD among patients with hemophilia. Unfortunately, there were no matched control subjects in this study, which weakens the comparisons.
Although mortality from CVD may be 50% lower in patients with hemophilia compared with the general population, most studies were not developed to assess cardiovascular risk. Furthermore, the average age of the population ranged between 44 and 54 years; consequently, the number of fatal cardiovascular events was low in these cohorts. Finally, in the registries, mortality was in most cases obtained by death certificates, which is clearly less reliable than autopsy. There is a need of prospective data on CVD and cardiovascular mortality in patients with hemophilia, especially because the life expectancy of the hemophilia population is increasing.
Cardiovascular risk factors in hemophilia
The lower cardiovascular mortality in patients with hemophilia may be caused by a beneficial cardiovascular risk profile. In clinical practice, patients with hemophilia are regularly controlled in a hemophilia treatment center, which could result in a more health-conscious lifestyle, such as a healthy diet and regular exercise. On the other hand, reduced mobility from arthropathy as a result of frequent bleeding could result in a higher prevalence of obesity, diabetes, and dyslipidemia. Several studies assessed the presence of hypertension in hemophilia. In a survey among Dutch patients with hemophilia, hypertension was doubled compared with the general population.14 In a Dutch case-control study, 57% of hemophilia A and B patients older than 18 years had hypertension, defined as a blood pressure >140/90 mm Hg compared with 37.5% in healthy controls.15 An analysis of 386 Dutch and 315 UK hemophilia patients older than 30 years showed that 49% of the hemophilia patients had hypertension compared with 40% in the general age-matched male population.16 A recent study from the United States found similar figures: prevalence of hypertension in patients with hemophilia older than 18 years was 49.1% compared with 31.7% in the general population.17 It is not completely clear why hypertension is more prevalent in patients with hemophilia. Interestingly, in these last 2 studies, hypertension seemed to be more prevalent in patients with a more severe type of hemophilia. Furthermore, in the US study, hypertension was associated with a decreased renal function and increased creatinine level.17 Although speculative, patients with hemophilia may suffer from (micro)bleeds in the kidneys, resulting in renovascular hypertension and fibrosis.18 This hypothesis needs further exploration.
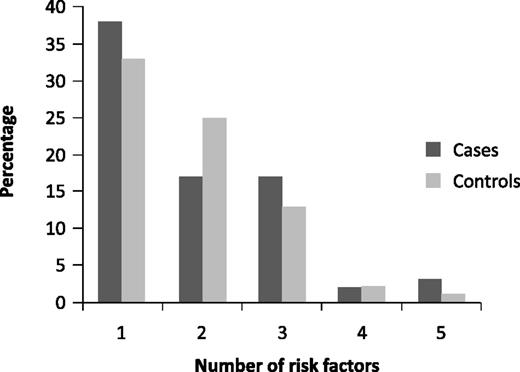
The prevalence of diabetes mellitus and smoking is not different between patients with hemophilia and the general population.16 Total cholesterol and high-density lipoprotein levels were lower in hemophiliacs in the Dutch and UK cohorts compared with the general population, especially in the patients with severe hemophilia.16 Fifty-six percent of the patients with hemophilia had total cholesterol levels <5.0 mmol/L and 32% had high-density lipoprotein levels <1.0 mmol/L compared with 32% and 8% in the general population, respectively. In the same study, 15% of the hemophiliacs were obese (body mass index >30 kg/m2) compared with 20% in the general population. Figure 2 shows the clustering of cardiovascular risk factors in patients with hemophilia and healthy controls15 and clearly demonstrates that many hemophilia patients have multiple risk factors for CVD. The expected 10-year mortality risk from CVD can be calculated with different scoring systems, such as the Systemic Coronary Risk Evaluation.19 This risk score is based on cardiovascular risk factors and estimates the total mortality from CVD and stroke. A score >10% confers a high risk, necessitating intervention. The percentage of hemophilia patients with such a high cardiovascular mortality risk score was 12% compared with 7% in the controls (P = .18).15 The mean 10-year QRISK 2-2011 score, a specific UK CVD risk score, was 8.9% (CI 8.1-9.8) in patients with hemophilia, higher than in the general population (6.7%, CI 6.1-7.2, P < .001).16
Clustering of cardiovascular risk factors in patients with hemophilia and matched control subjects.15
Clustering of cardiovascular risk factors in patients with hemophilia and matched control subjects.15
In conclusion, the CVD risk of patients with hemophilia is as least as high as in the general population, whereas hypertension is more common. Furthermore, patients with hemophilia may suffer from HIV or hepatitis C infection, conditions associated with an increased risk of CVD.20,21 In 2 registries, however, hepatitis C or HIV did not increase cardiovascular mortality in patients with hemophilia,10,11
Atherosclerosis in patients with hemophilia
Hemostasis not only contributes to clot formation, but is also involved in the process of atherosclerosis.22 Thrombin is the well-known key player in both fibrin formation and platelet activation. However, thrombin is also involved in atherogenic processes, such as endothelial dysfunction and barrier disruption, oxidative stress, apoptosis, inflammation, activation of platelets and leukocytes, and proliferation of smooth muscle cells, partly through protease-activated receptors 1 and 4.22,23 Patients with hemophilia, who have a decreased thrombin formation, may have reduced formation of atherosclerosis. Apolipoprotein E knockout (apoE−/−) mice that have accelerated atherosclerosis, developed less early-stage atherosclerotic lesions when they were also factor VIII–deficient.24 On the other hand, in mice with a low-density lipoprotein receptor null mutation, another accelerated atherosclerosis model, the protective effect of factor VIII deficiency was not observed.25 In humans, several earlier studies analyzed the presence of subclinical atherosclerosis by measuring the carotid and femoral intima media thickness (IMT) in patients with hemophilia or von Willebrand disease.26-29 Overall, there was no difference in the mean IMT of either the carotid artery (0.75 mm vs 0.74 mm) or femoral artery (0.75 mm vs 0.79 mm) in patients and age-matched healthy controls.8
Recently, 2 studies investigated whether hypocoagulability is associated with decreased atherogenesis by evaluating subclinical atherosclerosis and endothelial function in hemophilia patients and in matched unaffected controls.6,7 The first study, a multicenter study from The Netherlands and Belgium compared 51 hemophilia A and B patients with obesity, as a proxy for increased cardiovascular risk, with 47 normal weight hemophiliacs and healthy age-matched controls with (42) and without (50) obesity.6 The mean age of the subjects was 50 years and none had previous CVD. Carotid IMT was increased in obese (0.77 ± 0.22 mm) compared with nonobese subjects (0.69 ± 0.16 mm). When comparing obese hemophilia patients with obese controls, no difference in carotid IMT was apparent (IMT 0.78 ± 0.23 mm and 0.76 ± 0.22 mm, respectively, mean difference 0.02 mm, 95% CI −0.07 to 0.11, P = .67). The mean carotid IMT was not different in severe and moderate hemophilia patients compared with controls. Interestingly, 33% of the hemophilia patients had an atherosclerotic plaque in the carotid artery compared with 25% of the controls (P = .25). In the second study, from The Netherlands, 69 hemophilia A and B patients were analyzed, of which 9 had previous CVD. The mean age was 52 years and the mean carotid IMT was 0.80 mm, comparable with age-specific reference values. In both studies, there was no difference between hemophilia A and B. Finally, subclinical atherosclerosis was analyzed by coronary artery calcification with multidetector-row computed tomography in 42 men, ≥59 years, with severe or moderate hemophilia A (factor VIII <5%) and 613 nonhemophilic men.30 None of the subjects had a history of CVD and mean age of the study population was 66.5 years. The results showed that 24% of the subjects in both groups had severe calcification of the coronary arteries, despite the fact that patients had severe or moderate hemophilia, which again challenges the protective effect of factor VIII deficiency on the development of atherosclerosis.
In summary, these recent studies clearly show that patients with hemophilia have the same degree of atherosclerosis burden as the general population. How does this correspond with a reduced CVD and cardiovascular mortality in hemophiliacs with the apparent lack of protection against atherosclerosis burden? The experimental work may point to a possible explanation. Although the protective effect of factor VIII deficiency on atherosclerosis development in mice is inconsistent, the opposite phenotype of hypercoagulability appears to stimulate atherosclerosis, not only by affecting plaque burden, but also by affecting plaque phenotype. Thus, in mice with an apoE−/− background crossed with mice with a mutation in the thrombomodulin gene, causing a defect in protein C activation, increased atherosclerosis with a more stable plaque phenotype was seen in relatively young mice,31 whereas in another study in older mice of the same genetic background, similarly advanced atherosclerosis was associated with clear evidence of instability.32 These findings suggest that although, based on the burden of atherosclerosis, hypercoagulability may seem to be of equal impact, the actual plaque phenotype may markedly differ. For genetic hypocoagulability, there are insufficient data to show effects on plaque phenotype. Interestingly, pharmacological treatment of mice on an apoE−/− background with the selective thrombin inhibitor dabigatran or with 1 of the factor Xa inhibitors melagatran or rivaroxaban clearly indicates that targeting 1 coagulation protease diminishes atherogenic features of vascular endothelium and reduces plaque burden.29,32-34 Collectively, one may postulate that in the case of hemophilia, where the lack of factor VIII reduces basal levels of thrombin generation, this may (favorably) affect the plaque phenotype rather than the plaque load, which is driven by stronger factors including hypertension. Both the strength and the direction of the effect of hypocoagulability will be influenced by age, gender, genetic background, and cardiovascular risk factors. This might also explain that although the extent of atherosclerosis assessed by IMT measurement may be increased in hemophilia patients, increased stability of the plaque lesions may still have a net beneficial effect with regard to cardiovascular mortality. Obviously, this postulate needs further experimental and clinical testing because the lower risk of CVD may also be explained by a reduced formation of occluding thrombi.
Clinical consequences of cardiovascular risk in patients with hemophilia
In today’s clinical practice, we see more and more hemophilia patients with cardiovascular events. The treatment of these events is extremely challenging, as described previously in this journal35 because the core treatment consists of antiplatelet therapy, which will further increase the bleeding tendency of the hemophilia patient. Next, cardiologists are unfamiliar with hemophilia, and vice versa, hematologists usually do not treat patients with acute CVD. This necessitates multidisciplinary teams and the development of guidelines.36
But above all and regardless of the underlying mechanisms, prevention of CVD in adult hemophilia patients is crucial. Physicians who treat these patients should actively look for cardiovascular risk factors, most notably hypertension, and promote a healthy lifestyle. Treatment of the risk factors should follow international guidelines, where treatment goals are clearly defined.37,38
Conclusions
Patients with hemophilia may have a lower cardiovascular mortality, but have the same high prevalence of subclinical atherosclerosis as the general population. Furthermore, of all cardiovascular risk factors, hypertension is more common in hemophilia, which not only increases CVD, but also the risk of intracerebral bleeding. Cardiovascular prevention with medication and a healthy lifestyle is mandatory and begins with an active search for risk factors. Furthermore, more information is needed on the long-term CVD risk of adult hemophilia patients, atherosclerotic plaque stability, and on the optimal treatment of cardiovascular events.
Authorship
Contribution: P.W.K. and H.t.C. wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pieter W. Kamphuisen, Department of Vascular Medicine, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: p.w.kamphuisen@umcg.nl.