In this issue of Blood, Liu et al demonstrate that CD138+ myeloma plasma cells produce and secrete sonic hedgehog (SHH) protein, which is used in an autocrine fashion to maintain cell viability and proliferation.1
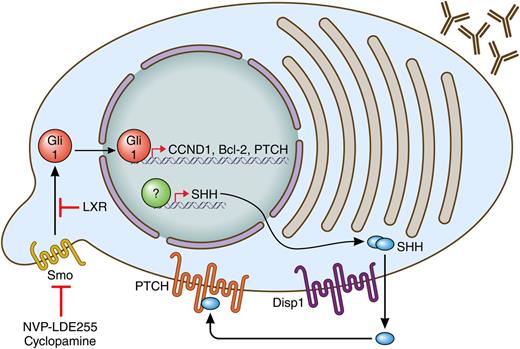
Myeloma plasma cells, as depicted by antibody secretion, produce SHH through an unknown mechanism. They can secrete it via Disp1 and then use it through their PTCH receptors to allow for activation of SMO (at a cilia) to activate Gli1. Gli1 regulates proliferation and survival through target genes CCND1 and Bcl-2 and activates a negative feedback loop through he activation of PTCH expression. Targeting the pathway can be achieved through SMO antagonists (cyclopamine, NVP-LDE255) or agonists of liver X receptors (LXRs). Professional illustration by Patrick Lane, ScEYEnce Studios.
Myeloma plasma cells, as depicted by antibody secretion, produce SHH through an unknown mechanism. They can secrete it via Disp1 and then use it through their PTCH receptors to allow for activation of SMO (at a cilia) to activate Gli1. Gli1 regulates proliferation and survival through target genes CCND1 and Bcl-2 and activates a negative feedback loop through he activation of PTCH expression. Targeting the pathway can be achieved through SMO antagonists (cyclopamine, NVP-LDE255) or agonists of liver X receptors (LXRs). Professional illustration by Patrick Lane, ScEYEnce Studios.
Hedgehog (Hh) is an evolutionarily conserved signaling pathway originally described in Drosophila.2 It is an important regulator of development and stem cell fate. Hh signaling is initiated when the Hh ligands (SHH), desert hedgehog, or Indian hedgehog (IHH) bind to Patched (PTCH) protein on the surface of the responder cell. When PTCH is not bound to ligand, it inhibits the Smoothened (SMO) membrane protein; however, once PTCH is bound by an Hh ligand, SMO can signal into the cell. The signaling results in the activation of the Gli transcription factors, which can then activate cell cycle genes such as CCND1 and antiapoptotic genes, including Bcl-2 as well as PTCH to form a negative feedback loop (see figure). Dysregulation of the pathway is also observed in several tumor types, including basal cell carcinoma and medulloblastoma. This has prompted investigation of the pathway in other cancers, including multiple myeloma (MM). A potential role for the Hh pathway in myeloma was first established when several pathway genes were shown to be upregulated in CD138-purifed cells from patients with monoclonal gammopathy of undetermined significance and MM when compared with normal plasma cells.3 Along with the development of inhibitors of the pathway, this prompted further investigation of the role of Hh in myeloma and its potential as a therapeutic target in the disease. These studies seem to raise as many questions as they answer. Specifically, Which myeloma cells are the Hh-responsive cells? and Where is the Hh ligand coming from?
While several reports, including that by Liu et al1 have attempted to address the first question, it does not appear that a consensus has been reached. Peacock et al4 concluded that the CD138–/CD19+ cells that had tumor-initiating activity were the Hh-responsive cells and, consistent with other stem-cell populations, these cells were maintained in a less differentiated state by Hh signaling. In that study, CD138+ cells did not respond to Hh. In contrast, at about the same time, Dierks et al5 determined that purified CD138+ myeloma cell survival was maintained by a stromal cell line that expressed IHH. Stromal support was blocked by the addition of the SMO inhibitor cyclopamine. Thus CD138+ myeloma plasma cells could also be Hh responsive and, consistent with that possibility, a more recent study confirmed these findings and demonstrated preclinical activity of the newer SMO inhibitor NVP-LDE225.6 Interestingly, the latter study demonstrated that myeloma cells, in addition to responding to SHH, also expressed elevated SHH levels. However, on the basis of several correlative observations, it was concluded that the primary source of SHH in myeloma was bone marrow stromal cells (BMSCs).
In addition to confirming the expression of SHH in CD138+ samples,6 the Liu et al study demonstrates that MM cells are capable of secreting SHH. As part of this demonstration, the authors showed that Dispatched homolog 1 (Drosophila) (Disp1), a mediator of SHH secretion,7 is expressed in myeloma cells and is necessary for SHH secretion.1 This is important because it allows the authors to confirm that SHH has autocrine effects in CD138+ MM cells. The authors then show, through gain and loss of function experiments as well as through the use of inhibitors, that SHH produced by MM cells is important for cell survival and proliferation and that it also plays a role in therapeutic responses both in vitro and in vivo. These latter findings are consistent with the earlier study and point to the potential utility of SMO antagonists in combination therapy in myeloma.6 The possibility of using liver X receptor agonists as downstream inhibitors of Hh signaling as an alternative approach to block this pathway was also recently suggested and could provide a means to target this pathway when SMO antagonists are ineffective.8
Like most interesting studies, this one raises new questions that need to be addressed, most notably, Why have there been discrepancies in the findings regarding which cells are producing SHH? and Which cells are responsive to Hh signals? As for which cells produce the SHH, the Liu et al study clearly demonstrates that myeloma cells can be the source; however, it also argues that BMSCs are unlikely to be a source. Earlier studies pointing to BMSCs did not take into account the ratio of stromal cells to myeloma cells and therefore may have overrepresented the role of stroma. If the primary source of SHH is autocrine, then changes in this pathway are less likely to be a marker of stromal independence.
Regarding the use of Hh signaling in MM, we have one study stating that only the CD138– cells are involved,4 a second stating that both CD138+ and CD138– cells express the appropriate proteins for Hh signaling,6 and now a third that claims it is only the CD138+ cells.1 Some of these issues are probably technical because different cell types, different growth conditions, and different functional assays are used throughout the studies. If one assumes that both CD138+ and CD138– are responsive to Hh signaling, then the most likely model would be that Hh signaling has different consequences in cells at different stages of differentiation. In the case of the less mature cells, it appears to be important in self-renewal but not in survival because the cells differentiate when Hh signaling is inhibited. In the differentiated cells, the function appears to be more of a survival and proliferative control as evidenced by changes in expression of Bcl-2, CCND1, and differing responses to therapy. If this is the case, then targeting this pathway clinically becomes more important because it may function in the killing of the plasma cells as well as depleting a tumor initiating pool.
Conflict-of-interest disclosure: The author declares no competing financial interests.