Abstract
Immunotherapy has emerged as a viable clinical strategy to harness endogenous antitumor T-cell immunity. Lenalidomide is an oral immunomodulatory drug that repairs antitumor T-cell function and is showing efficacy in ongoing chronic lymphocytic leukemia (CLL) and lymphoma clinical trials. This article focuses on advances in our understanding of its mechanism of action in the tumor microenvironment and provides a clinical update in CLL. Challenges associated with this drug and its potential use in the targeted drug treatment era are discussed.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common B-cell malignancies in adults, characterized by an accumulation of monoclonal CD5+ mature B cells in lymphoid tissues and the peripheral blood. Clonal expansion and invasive migration typically causes the lymph nodes, spleen, and the bone marrow to become infiltrated with tumor. Current standard therapy combines chemotherapy with an anti-CD20 monoclonal antibody (mAb) (chemoimmunotherapy [CIT]). Although highly potent, CIT induces substantial toxicity and is not curative, with nearly all patients eventually relapsing. Recent advances using kinase inhibitors (eg, ibrutinib and idelalisib) that target B-cell receptor (BCR) signaling indicate an exciting shift toward a nonchemotherapy treatment era (reviewed by Jones and Byrd1 ). Present indications suggest these drugs are not producing many complete responses and should be taken continuously to avoid relapse. Unanswered questions include whether long-term persistent disease and prolonged therapy promote drug-resistant variants through clonal evolution and/or activation of compensatory oncogenic signaling in CLL. Ibrutinib resistance has already been detected in genetically high-risk patients,2 highlighting the necessity to identify combinatorial therapy using agents with distinct mechanism of action (MOA).
In addition to genome alterations, CLL exhibits another dimension of complexity: leukemic cells are nurtured and protected from anticancer therapies by a variety of resident and recruited ostensibly normal cells that constitute the tumor microenvironment (TME) in lymphoid organs. Nonmalignant components of the TME include: mesenchymal stromal/stem cells (MSCs), endothelial cells, tumor-associated macrophages (TAMs or “nurse-like cells” [NLCs]), dendritic cells (DCs) and T cells. A recent breakthrough in cancer therapeutics has been the use of immunotherapies (immune checkpoint blockade) that target mechanisms of T-cell evasion by tumors.3 In this review, we focus on the immunomodulatory drug (IMiD) lenalidomide which activates antitumor T-cell activity and is showing clinical activity in ongoing CLL clinical trials. Lenalidomide (Revlimid) is a derivative of thalidomide that is US Food and Drug Administration (FDA) approved for the treatment of multiple myeloma, myelodysplastic syndromes, and mantle cell lymphoma. Remarkably, in contrast to the BCR inhibitor drugs, IMiDs exemplify successful bedside-to-bench research, in that their clinical effectiveness was known before recent MOA data emerged that help explain their pleotropic effects in the TME.
The TME in CLL
Active crosstalk between leukemic cells and nonmalignant cells in the TME plays a critical role in activating tumor migration, survival, proliferation, and fostering immune privilege (reviewed by Burger and Gribben4 ).
Briefly, CLL cells migrate into their niches via prosurvival CXC chemokine ligand 12 (CXCL12)- and CXCL13-chemokine gradients released by MSCs, NLCs, follicular helper CD4+ T cells (TFH), and follicular DCs (FDCs). In the TME, MSCs and endothelial cells induce signal transducer and activator of transcription 3 (STAT3) and nuclear factor–κB (NF-κB)–mediated prosurvival signaling in CLL cells. Monocyte-derived NLCs supply pro-CLL survival signals and resemble proangiogenic and immunosuppressive TAMs.4 Notably, CLL-stroma crosstalk is bidirectional and stromal cells in turn also become activated by leukemic cells. CLL-secreted soluble factors also activate receptors on MSCs leading to AKT activation, proliferation, and secretion of proangiogenic vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF).5
BCR activation in the TME has emerged as a central oncogenic pathway6 essential for CLL survival and proliferation. BCR engagement likely involves extrinsic autoantigens and/or microbial antigens, as well as autoreactive autonomous activation.6 Signals from the BCR and the tissue TME (cellular and molecular interactions) converge on several key intracellular signaling pathways including the phosphatidylinositol 3-kinase (PI3K)–AKT axis.4 FDCs and TFH cells are specialized reservoirs of intact antigen and cognate B-cell help, respectively, and likely contribute to BCR activation.
Intriguingly, subverted CD4+ T cells support CLL cells in the TME, whereas antileukemic CD8+ T cells are suppressed. Activated CD4+ T cells and TFH cells can provide CD40L costimulation and prosurvival T helper 2 (Th2) cytokines (interleukin-4 [IL-4], IL-6, IL-21) that trigger extracellular signal-regulated kinase (ERK), STAT-3/-6, and NF-κB signaling and leukemic proliferation. However, CLL cells also subvert T cells to avoid immune destruction by inducing defective immune synapsis.7 Immune synapses (formed at T-cell–APC interfaces during antigen recognition) have a master role in T-cell activation and polarized delivery of effector molecules. CLL cells express low levels of costimulatory molecules while coopting multiple immune checkpoint coinhibitory pathways including PD-L1 that deliver an inhibitory signal into PD1+ T cells to actively suppress synapses and secretion of IL-2 and lytic granules.8 Additional immune evasion mechanisms include leukemic-derived factors that suppress NK-cell lytic activity and induction of regulatory T cells (Tregs).4
How does lenalidomide target malignant B cells?
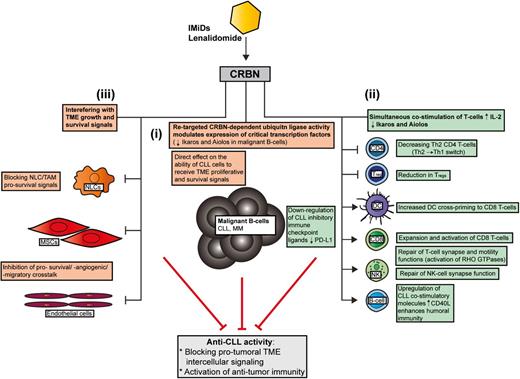
Seminal breakthrough studies have recently identified that IMiDs (thalidomide, lenalidomide, and pomalidomide) bind to the protein target cereblon (CRBN), altering an E3 ligase complex that modulates expression of critical transcription factors. In the absence of IMiDs, CRBN-directed E3 ubiquitin ligase activity is directed to CRBN itself and possibly other specific proteins that are tagged for proteasome-mediated degradation. IMiDs bind to CRBN and modulate its substrate recognition, preventing autoubiquitylation. Three independent studies have simultaneously revealed that IMiDs retarget CRBN-dependent ligase activity toward the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) and induce their proteasomal degradation.9-11 Their decreased abundance leads to loss of viability in myeloma B cells. Ikaros and Aiolos are highly expressed in CLL cells and there is evidence supporting their role in regulating survival signaling.12,13 Testing whether the ability of lenalidomide to downregulate these transcription factors in lymphocytes contributes to both direct and indirect anti-CLL activity will be of great interest. Lenalidomide is not directly cytotoxic to CLL cells in vitro, but does alter CLL-TME protumoral signals. Direct anti-CLL effects include inhibition of TME-induced proliferation in a CRBN/p21-dependent manner that is associated with reduced expression of IKZF1 and IKZF3 in common with myeloma (Figure 1i).14
How lenalidomide targets the CLL TME. In contrast to conventional targeted drugs, lenalidomide seems to exert most of its anti-CLL activity by interfering with protumoral TME interactions rather than directly targeting prosurvival signaling in the tumor clone itself. Recent MOA data have shown that the IMiD lenalidomide binds to the protein CRBN, which modulates its substrate recognition and augments the ubiquitylation and degradation of critical transcription factors in lymphocytes. Ikaros (IKZF1) and Aiolos (IKZF3) are the downregulated targets in MM that cause direct malignant B-cell toxicity. Whether these transcription factors are targeted by lenalidomide in the diverse cellular components of the CLL TME remains to be investigated. This IMiD retargeted CRBN-dependent ubiquitin ligase activity simultaneously alters malignant B-cell and nonmalignant T-cell function. (i) Emerging data have shown that a retargeted CRBN complex directly inhibits TME-mediated proliferation signaling in CLL B cells that is associated with reduced expression of IKZF1 and IKZF3 in common with myeloma. (ii) Importantly, degradation of Ikaros and Aiolos in T cells stimulates IL-2 secretion that enhances immune function. Lenalidomide has been shown to effectively reverse tumor-induced immune suppression/privilege. Immunomodulatory MOA includes: promoting a Th2 to Th1 CD4+ T-cell switch, downregulating immunosuppressive signaling axes (eg, PD:L1:PD1) while enhancing costimulatory molecules that positively regulate antitumor CD8+ T-cell function and the induction of humoral immunity (residual normal B cells). CRBN-dependent activation of RHO GTPase activation signaling and cytoskeletal signaling repairs T-cell lytic immune synapse and motility function. (iii) Lenalidomide also modulates tumor-educated stromal cells (TAMs/NLCs and MSCs) in the TME that block essential survival signals for the expanding malignant B-cell clone. MM, multiple myeloma.
How lenalidomide targets the CLL TME. In contrast to conventional targeted drugs, lenalidomide seems to exert most of its anti-CLL activity by interfering with protumoral TME interactions rather than directly targeting prosurvival signaling in the tumor clone itself. Recent MOA data have shown that the IMiD lenalidomide binds to the protein CRBN, which modulates its substrate recognition and augments the ubiquitylation and degradation of critical transcription factors in lymphocytes. Ikaros (IKZF1) and Aiolos (IKZF3) are the downregulated targets in MM that cause direct malignant B-cell toxicity. Whether these transcription factors are targeted by lenalidomide in the diverse cellular components of the CLL TME remains to be investigated. This IMiD retargeted CRBN-dependent ubiquitin ligase activity simultaneously alters malignant B-cell and nonmalignant T-cell function. (i) Emerging data have shown that a retargeted CRBN complex directly inhibits TME-mediated proliferation signaling in CLL B cells that is associated with reduced expression of IKZF1 and IKZF3 in common with myeloma. (ii) Importantly, degradation of Ikaros and Aiolos in T cells stimulates IL-2 secretion that enhances immune function. Lenalidomide has been shown to effectively reverse tumor-induced immune suppression/privilege. Immunomodulatory MOA includes: promoting a Th2 to Th1 CD4+ T-cell switch, downregulating immunosuppressive signaling axes (eg, PD:L1:PD1) while enhancing costimulatory molecules that positively regulate antitumor CD8+ T-cell function and the induction of humoral immunity (residual normal B cells). CRBN-dependent activation of RHO GTPase activation signaling and cytoskeletal signaling repairs T-cell lytic immune synapse and motility function. (iii) Lenalidomide also modulates tumor-educated stromal cells (TAMs/NLCs and MSCs) in the TME that block essential survival signals for the expanding malignant B-cell clone. MM, multiple myeloma.
How does lenalidomide simultaneously modulate immune/stromal cells in the TME?
Importantly, Ikaros and Aiolos are repressors of the IL-2 promoter, and their degradation in response to IMiDs explains enhanced costimulation and IL-2 secretion in T cells.11 Thus, the ability of IMiDs to target CRBN and the ubiquitin proteasome system modulates the expression of critical transcription factors that costimulate T cells, while degrading B-cell function simultaneously (Figure 1). It should be noted that the CRBN complex is likely to have distinct sets of targets in different healthy and cancer cell types. Moreover, proteins that are degraded (polyubiquitylated), compartmentalized (monoubiquitylated), or sequestered by the CRBN complex, or differentially released and stabilized by IMiDs, are likely to contribute to their biological activity.15
IMiDs increase the DC cross-priming,16 expansion, and activation of CD8+ T cells while decreasing activated CD4+ T-cell–derived cytokines,17 promoting Th1 T-cell differentiation, and polarizing Th2 T cells to a Th1 phenotype (interferon-γ [IFN-γ], tumor necrosis factor-α [TNF-α]).1 This Th2 to Th1 switch is linked to increased expression of the transcription factor T-bet.1
Lenalidomide repairs the tumor-induced T-cell immune synapse defect in CLL and lymphoma by increasing the assembly and activity of cytoskeletal signaling molecules including protein kinase C-θ (PKC-θ), WASp, and RHO GTPase CDC42 to the synapse, allowing effective T-cell receptor (TCR)/CD28 signal transduction and directional secretion of lytic granules.7,8 IMiD-mediated repair of NK-cell lytic synapse formation is also emerging.1,4 Lenalidomide also rescues T-cell motility in CLL by normalizing RAC1, RHOA, and CDC42 activity levels. Importantly, knockdown of CRBN blocks lenalidomide repair of T-cell function.18 Taken together, enhancement of T-cell–mediated responses by lenalidomide is linked to a retargeted CRBN complex and altered transcription and activation of critical RHO GTPases.8
Lenalidomide also alters RHO GTPase activation signaling that degrades malignant B-cell function (migration capability) and TME interactions (blocks prosurvival signaling interactions with NLCs).19,20 Upregulation of costimulatory molecules such as CD80 and CD86 on CLL cells by lenalidomide contributes to the repair of T-cell synapses.7 Enhanced expression of CD40L on CLL cells by lenalidomide promotes immunoglobulin production by normal B cells21 that has been detected in long-term treatment responders. Thus, repair of the humoral defect may contribute to enhanced antitumor immunity. Lenalidomide downregulates the PD-L1:PD-1 immunosuppressive axis8 in CLL, lymphoma and myeloma, allowing T cells and NK cells to form lytic synapses with target tumor cells. Paradoxically, PD-1 positively regulates the suppressive activity of Tregs. Decreased PD-18 and transcription factor FOXP3 expression may explain how IMiDs reduce Tregs while activating CD8+ T cells17 (Figure 1ii).
CLL cells position themselves in close contact with endothelial cells in the TME.4 Lenalidomide inhibits bidirectional prosurvival crosstalk between endothelial cells and tumor cells including proangiogenic signals.22 Notably, plasma levels of proangiogenic VEGF and FGF were reduced in CLL patients who responded to therapy.22 IMiD inhibition of MSC-derived CXCL121 may interfere with the CXC chemokine receptor 4 (CXCR4)-CXCL12 migratory axis in the CLL-TME (Figure 1iii).
Clinical experience with lenalidomide
The initial dose of lenalidomide in CLL clinical trials was chosen based on experience in myeloma but this induced serious side effects including rapid tumor cell death (tumor lysis syndrome [TLS]) and acute inflammation (tumor flare reaction [TFR], that may be associated with immune-mediated clinical response). Therefore, a low initial dose with dose escalation has been applied in subsequent trials and has improved tolerability.
Published results discussed here are summarized and referenced in Table 1 (ongoing studies: supplemental Table 1, see supplemental Data available at the Blood Web site).23-41 When used as monotherapy, overall response rates (ORRs) have been up to 72% (mostly partial responses) for first-line therapy and 25% to 30% for fludarabine-refractory CLL. Lenalidomide has shown clinical activity in patients with high-risk features such as del(17p) (31%-38% ORR). Lenalidomide was shown to antagonize NK-cell killing of rituximab-treated CLL cells if both agents were used simultaneously.42 However, this has not translated to the clinic (likely due to drug scheduling) as results suggest improved efficacy and lower occurrence of TLS and TFR when combining rituximab or ofatumumab with lenalidomide.
Experience with lenalidomide in the consolidation/maintenance setting has been limited. Results from a phase 2 trial of CIT followed by lenalidomide showed that 24% of patients improved their quality of response with consolidation and some converted to minimal residual disease–negative status.41 These results are encouraging for an active placebo-controlled phase 3 trial of lenalidomide maintenance in minimal residual disease–positive patients post-CIT.
How to manage safety and toxicity?
The randomized ORIGIN trial that compared the safety and efficacy of front-line lenalidomide vs chlorambucil in elderly patients was halted by the FDA for safety concerns following an imbalance in the number of deaths in the lenalidomide treatment arm. This experience is in contrast to a trial reporting long-lasting efficacy with lenalidomide monotherapy25 and highlights the safety and toxicity challenges associated with trial management. Clinical experience suggests an individual component to how CLL patients tolerate lenalidomide, and identifying predictive pretreatment factors requires future research. Grade 3/4 neutropenia has been reported in 70% to 83% of treated CLL patients (Table 1). The cause of lenalidomide-induced neutropenia may be related to the downregulation of transcription factor PU.1,43 that can be alleviated with granulocyte colony-stimulating factor (G-CSF) treatment.
Lenalidomide-induced TFR can be effectively managed with anti-inflammatory drugs (eg, dexamethasone). Notably, the combination of lenalidomide with fludarabine and rituximab results in a dramatic reduction in TFR.
TLS has been reported in 0% to 4.5% of patients from phase 2 studies. Slow-dose escalation, close monitoring, and prophylaxis (eg, allopurinol) can effectively prevent TLS.
Identifying biomarkers of response or resistance to improve patient care
The immune synapse bioassay in combination with the lytic biomarker Granzyme-B8 has demonstrated utility as a knowledge-based T-cell–monitoring assay in the phase 2 trial of CIT followed by lenalidomide consolidation.41 CRBN’s central role as a target of IMiD immunomodulation supports its use as a biomarker. However, its multiple splice variants and potential lack of correlation between messenger RNA and protein highlights the challenges when assessing biomarkers. Furthermore, CRBN does not appear to be a good predictive marker in CLL as it exhibits uniform expression regardless of clinical response. In contrast, expression of GSK-3 has shown potential as a response biomarker.44,45 Future research on the modulation of CRBN-Ikaros/Aiolos signaling pathways by IMiDs in CLL may reveal effective biomarkers. Proof-of-principle results indicate that Aiolos could act as a biomarker for T-cell activation as in vivo lenalidomide treatment resulted in downregulation of this transcription factor.11
What is the future role of lenalidomide in the evolving treatment era?
Preclinical studies suggest that the PI3Kδ inhibitor idelalisib may antagonize the immune-modulating properties of lenalidomide including repair of the humoral defect.45 Whether this translates to the clinic remains to be seen. Combining immune checkpoint blockade (eg, anti-PD-1 mAb) with lenalidomide may enhance antitumor T-cell immunity in CLL and lymphoma as both agents block immunosuppressive signaling.8 However, such immunostimulatory trials will need careful monitoring for potential autoimmune reactions. Ibrutinib, via ITK (interleukin-2-inducible T-cell kinase)-mediated inhibition of proleukemic Th2 CD4+ T cells,46 may also have complementary immunomodulatory potential with IMiDs, enhancing Th1 CD4+ and CD8+ T-cell immunity. In contrast to lenalidomide, the Bcl-2–specific inhibitor ABT-199/GDC-0199 directly induces tumor lysis. Thus, combining lenalidomide/IMiDs with ibrutinib or other new agents with distinct MOA has strong preclinical rationale and future studies will be of great interest.
The online version of this article contains a data supplement.
Authorship
Contribution: A.G.R., A.P.K., S.H.T., and A.E. wrote the paper and approved the final version.
Conflict-of-interest disclosure: A.P.K. received research funding from Celgene. A.E. received research funding from, and provided consultancy for, Celgene. The remaining authors declare no competing financial interests.
Correspondence: Alan G. Ramsay, Department of Haemato-Oncology, Division of Cancer Studies, The Rayne Institute, King’s College London, 123 Coldharbour Lane, London, SE5 9NU, United Kingdom; e-mail: alan.ramsay@kcl.ac.uk.