Key Points
CLEC-2 activation induces proteolytic cleavage of GPVI and FcγRIIa but not itself.
CLEC-2 but not GPVI is detected on platelet-derived microparticles.
Abstract
The C-type lectin-like receptor CLEC-2 mediates platelet activation through a hem-immunoreceptor tyrosine-based activation motif (hemITAM). CLEC-2 initiates a Src- and Syk-dependent signaling cascade that is closely related to that of the 2 platelet ITAM receptors: glycoprotein (GP)VI and FcγRIIa. Activation of either of the ITAM receptors induces shedding of GPVI and proteolysis of the ITAM domain in FcγRIIa. In the present study, we generated monoclonal antibodies against human CLEC-2 and used these to measure CLEC-2 expression on resting and stimulated platelets and on other hematopoietic cells. We show that CLEC-2 is restricted to platelets with an average copy number of ∼2000 per cell and that activation of CLEC-2 induces proteolytic cleavage of GPVI and FcγRIIa but not of itself. We further show that CLEC-2 and GPVI are expressed on CD41+ microparticles in megakaryocyte cultures and in platelet-rich plasma, which are predominantly derived from megakaryocytes in healthy donors, whereas microparticles derived from activated platelets only express CLEC-2. Patients with rheumatoid arthritis, an inflammatory disease associated with increased microparticle production, had raised plasma levels of microparticles that expressed CLEC-2 but not GPVI. Thus, CLEC-2, unlike platelet ITAM receptors, is not regulated by proteolysis and can be used to monitor platelet-derived microparticles.
Introduction
Platelets play an essential role in limiting blood loss from the damaged vasculature, but can also block blood flow in diseased vessels through formation of an occluding thrombus.1 Platelet adhesion and activation are ordinarily triggered by exposure to subendothelial extracellular matrix proteins. Initial adhesion to the damaged vessel wall is regulated through the interaction of the glycoprotein (GP)Ib-V-IX complex with von Willebrand factor immobilized on exposed collagen fibers. Platelet activation and recruitment to a growing thrombus is regulated by the collagen receptor GPVI and the platelet-secreted agonists adenosine diphosphate and thromboxane (Tx)A2.2 Thrombin, generated through the coagulation cascade, further activates platelets and strengthens clot formation by converting fibrinogen into fibrin. Adenosine diphosphate, TxA2, and thrombin signal via heterotrimeric G protein-coupled receptors. The collagen receptor GPVI signals through an immunoreceptor tyrosine-based activation motif (ITAM) via its associated subunit, the FcRγ chain. The signals from G protein-coupled receptors and GPVI synergize to mediate activation of integrin αIIbβ3-dependent platelet aggregation.2
Human platelets express 2 other receptors that signal through a closely related pathway to that of GPVI: the low affinity immunoglobulin ITAM receptor FcγRIIa and the podoplanin hemITAM receptor C-type lectin-like receptor 2 (CLEC-2). FcγRIIa signals through a single ITAM in its cytosolic domain and is a critical mediator of platelet activation in immune thrombocytopenia,3-5 heparin-induced thrombocytopenia,6 bacterial infection,7,8 and cancer.9 CLEC-2, a type II transmembrane protein, signals via a single YxxL sequence known as a hemITAM and is the receptor for the type I transmembrane GP podoplanin, which is widely expressed outside of the vasculature, including lymphatic endothelial cells, type 1 lung alveolar cells, lymph node stromal cells, and the choroid plexus epithelium. Podoplanin is also present on inflammatory macrophages10,11 on a subset of activated T-helper (Th)17 cells.12,13 The function of CLEC-2 in hemostasis is, however, unclear, with reports indicating that it either plays a minor role14,15 or that it plays no role.16 More recently, CLEC-2 has been shown to play a vital collaborative role with GPVI in thrombosis.17
There is increasing recognition that platelet (hem)ITAM receptors play a pivotal role in processes beyond hemostasis. Platelet-specific deletion of CLEC-2, or deletion of one of its downstream signaling proteins, Syk, SLP-76, or PLCγ2, leads to a number of developmental problems including blood-lymphatic mixing in midgestation.18-20 GPVI and CLEC-2 are also required for the maintenance of vascular integrity at sites of inflammation. Mice with reduced platelet counts and deficiency in GPVI and CLEC-2 exhibit severe bleeding following inflammatory challenge, and this is independent of the major platelet receptors involved in hemostasis including integrin αIIbβ3.21 In patients with rheumatoid arthritis, GPVI signaling amplifies inflammation through collagen-dependent platelet microparticle production.22
(hem)ITAM receptors signal through Src and Syk tyrosine kinases. Src family kinases and/or Syk phosphorylate the conserved YxxL motifs, which allow Syk to bind to 2 phosphorylated tyrosines via its tandem SH2 domains. Activation of Syk in this way gives rise to a signaling cascade that triggers PLCγ2 and Ca2+ mobilization, generation of TxA2, integrin activation, and granule secretion.23 Activation of GPVI or FcγRIIa is also associated with extracellular metalloproteinase-mediated ectodomain shedding of GPVI24 and intracellular calpain-mediated cleavage of FcγRIIa, resulting in deletion of the ITAM domain.25 Significantly, activation of either receptor results in proteolysis of the other, and in both cases, this is dependent on Src and Syk activation.25,26
In the present study, we generated monoclonal antibodies (mAbs) to human CLEC-2 and used one of these, AYP1, to measure the level of CLEC-2 in resting and stimulated human platelets and on microparticles. We show that in contrast to GPVI and FcγRIIa, CLEC-2 is not regulated by proteolysis on activation or in response to stimulation of GPVI and FcγRIIa and that CLEC-2 but not GPVI is expressed on microparticles derived from activated platelets. The physiological significance of these findings is discussed.
Materials and methods
Antibodies and other materials
A recombinant extracellular fragment of human CLEC-2 (amino acids 68-229) was produced as previously described27 and used to raise mouse α-human CLEC-2 mAbs AYP1 and AYP2 (IgG1). A detailed description of other antibodies and materials used can be found in the supplemental Methods available on the Blood Web site.
Patients
Citrated blood samples from healthy individuals and from patients with rheumatoid arthritis were obtained with informed consent approved by the local ethics committee (ERN_11-0175 and 071Q270612, respectively). All patients with rheumatoid arthritis satisfied the 1987 American College of Rheumatology criteria for rheumatoid arthritis.28
Cell isolation and culture, protein deglycosylation, western blots, and measuring soluble GPVI
A detailed description can be found in the supplemental Methods.
Microparticle preparation
For analysis of microparticles, platelet-free plasma (PFP) was collected from citrated venous blood by double centrifugation at 2500g for 15 minutes.29 Microparticles within PFP were used for subsequent studies. Microparticles from activated platelets were collected by incubating washed platelets with indicated agonists for 1 hour at 37°C, followed by double centrifugation at 2500g for 15 minutes. Microparticles from megakaryocyte cultures (day 12) or from platelet concentrates (day 5) were collected using the same centrifugation protocol. Freshly prepared microparticles were analyzed by flow cytometry.
Flow cytometric analysis
Platelets and microparticles were analyzed by flow cytometry using an Accuri C6 flow cytometer (BD Biosciences). Platelets were characterized by forward scatter and side scatter. Appropriate antibodies, as indicated in the figure legends, were added and incubated for 15 minutes at 37°C. A total of 10 000 events were analyzed for each sample. Microparticles were characterized using an allophycocyanin-conjugated antibody against integrin αIIb (CD41a; BD Biosciences) against an isotype-matched control. The threshold was set on fluorescence (FL4) to exclude background noise. CD41+ microparticles were then analyzed for surface expression of CLEC-2 and GPVI using Alexa Fluor 488-conjugated AYP1 and 1G5, respectively, and appropriate isotype-matched controls. Antibodies were centrifuged at 18 000g for 5 minutes to remove protein aggregates prior to assay. All other reagents were filtered with a 0.22-µM filter.
Leukocytes were analyzed using a FACSverse flow cytometer (BD Biosciences). Antibody incubations were on ice for 20 minutes. Peripheral blood mononuclear cells and polymorphonuclear cells were first discriminated by their forward scatter and side scatter characteristics. Live cells were selected based on negative staining for Sytox Red followed by selection for negative staining of CD41. An antibody against CD45 was used as a general marker for leukocytes. The following markers were used to distinguish leukocyte subsets: CD3 (T cell), CD11c (dendritic cell), CD14 (monocyte), CD16 (neutrophil), and CD19 (B cell). The leukocyte subsets were analyzed for surface expression of CLEC-2 by Alexa Fluor 488-conjugated AYP1 relative to isotype-matched controls.
Statistical analysis
Data are means ± standard error of the mean, unless stated otherwise. Statistical analysis was performed using GraphPad Prism 5 (San Diego, CA) using the Mann-Whitney test. P < .05 was considered significant.
Results
Characterization of mouse mAbs against human CLEC-2
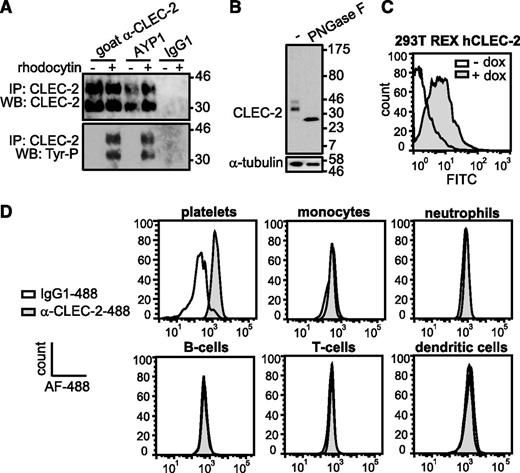
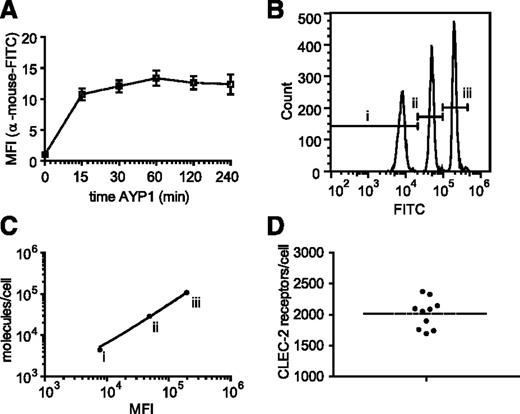
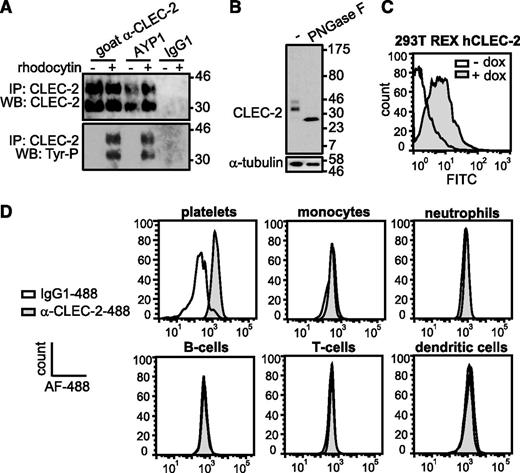
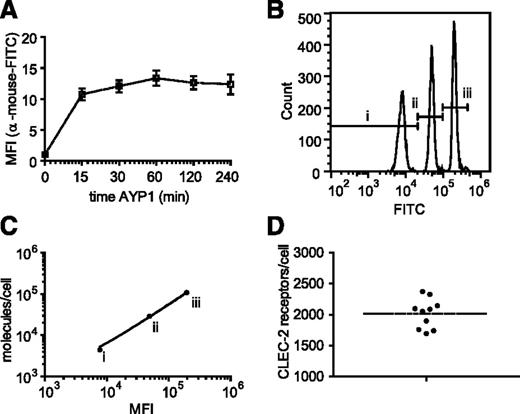
The extracellular domain of human CLEC-2 was used to generate mAbs to the C-type lectin receptor. One of these antibodies, AYP1 (IgG1), immunoprecipitated a 32- and 40-kDa doublet from lysates of control and rhodocytin-stimulated human platelets (Figure 1A). A second mouse α-human CLEC-2 mAb, AYP2 (IgG1), recognized the 2 bands by western blot, which correspond by molecular mass to differential glycosylated forms of human CLEC-2 described previously30 (Figure 1A-B). AYP1 did not detect CLEC-2 by western blot, indicating that the antibody binds to a conformational epitope that is lost during the denaturing conditions of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; data not shown). Probing for tyrosine phosphorylation demonstrated that AYP1 immunoprecipated both nonphosphorylated and phosphorylated forms of CLEC-2 (Figure 1A). AYP1 but not AYP2 recognized surface expressed CLEC-2 when analyzed by flow cytometry as shown using doxycycline-treated 293T Rex cells (Figure 1C).31 AYP1 also detected CLEC-2 on human platelets by flow cytometry but did not detect expression on monocytes, neutrophils, T and B cells, or dendritic cells in whole blood (Figure 1D; gating strategy described in supplemental Figure 1A). Analysis of leukocytes with a commercially available polyclonal antibody against CLEC-2 confirmed these findings (supplemental Figure 1B). This restricted distribution of CLEC-2 agrees with the BioGPS database (supplemental Figure 2) and shows that the C-type lectin receptor is selectively expressed on platelets in human blood. We used an established flow cytometry assay32 to determine the level of expression of CLEC-2 in healthy individuals. The receptor was present at 2016 ± 239 (mean ± standard deviation) copies per platelet (Figure 2A-D). This is within the same order of magnitude as the other platelet ITAM receptors GPVI (1250-9600)32-34 and FcγRIIa (1000-4000).35
AYP1 recognizes CLEC-2 on human platelets. (A) Washed platelets were incubated for 5 minutes at 37°C under stirring conditions in the absence and presence of 100 nM rhodocytin, followed by lysis and immunoprecipitation with 2 µg/mL of goat α-human CLEC-2 (previously characterized antibody), AYP1, or IgG1 coupled to protein-G sepharose. Proteins were separated on a 12% SDS-PAGE gel under reducing conditions, transferred onto a polyvinylidene difluoride membrane, and probed with AYP2. Membranes were subsequently stripped and reprobed with α-phosphotyrosine antibody 4G10. (B) Deglycosylation reduces CLEC-2 to a single band. Lysates of resting platelets were incubated in the absence or presence of peptide-N-glycosidase F (PNGase F), probed with AYP2, and stripped and reprobed with an antibody against α-tubulin. (C) Analysis of AYP1 binding to 293T Rex cells with doxycycline-inducible protein expression of CLEC-2. Cells were incubated in the absence or presence of 1 µg/mL of doxycycline for 24 hours, and AYP1 binding was determined by flow cytometry using a fluorescein isothiocyanate (FITC)-conjugated sheep α-mouse secondary antibody. (D) Flow cytometric analysis of CLEC-2 on platelets and leukocytes. Platelets and leukocytes were isolated and incubated with saturating concentrations of either Alexa Fluor-488 (AF-488) conjugated α-CLEC-2 antibody AYP1 or isotype-matched control for 30 minutes at room temperature (platelets) or on ice (leukocytes) and analyzed immediately. Leukocyte subset discrimination is described in the supplemental Methods and the gating strategy is shown in supplemental Figure 1A. Data are representative of ≥3 independent experiments.
AYP1 recognizes CLEC-2 on human platelets. (A) Washed platelets were incubated for 5 minutes at 37°C under stirring conditions in the absence and presence of 100 nM rhodocytin, followed by lysis and immunoprecipitation with 2 µg/mL of goat α-human CLEC-2 (previously characterized antibody), AYP1, or IgG1 coupled to protein-G sepharose. Proteins were separated on a 12% SDS-PAGE gel under reducing conditions, transferred onto a polyvinylidene difluoride membrane, and probed with AYP2. Membranes were subsequently stripped and reprobed with α-phosphotyrosine antibody 4G10. (B) Deglycosylation reduces CLEC-2 to a single band. Lysates of resting platelets were incubated in the absence or presence of peptide-N-glycosidase F (PNGase F), probed with AYP2, and stripped and reprobed with an antibody against α-tubulin. (C) Analysis of AYP1 binding to 293T Rex cells with doxycycline-inducible protein expression of CLEC-2. Cells were incubated in the absence or presence of 1 µg/mL of doxycycline for 24 hours, and AYP1 binding was determined by flow cytometry using a fluorescein isothiocyanate (FITC)-conjugated sheep α-mouse secondary antibody. (D) Flow cytometric analysis of CLEC-2 on platelets and leukocytes. Platelets and leukocytes were isolated and incubated with saturating concentrations of either Alexa Fluor-488 (AF-488) conjugated α-CLEC-2 antibody AYP1 or isotype-matched control for 30 minutes at room temperature (platelets) or on ice (leukocytes) and analyzed immediately. Leukocyte subset discrimination is described in the supplemental Methods and the gating strategy is shown in supplemental Figure 1A. Data are representative of ≥3 independent experiments.
Quantification of platelet CLEC-2 expression. The number of surface copies of CLEC-2 per platelet was determined using the mouse α-human CLEC-2 antibody AYP1 and the Platelet Calibrator Kit from Biocytex. (A) Washed platelets were preincubated with 2.5 µg/mL AYP1 (saturating concentration) at room temperature for indicated times and subsequently incubated with a FITC-conjugated α-mouse antibody for another 15 minutes. Flow cytometric analysis shows that binding of AYP1 remains stable over the tested time period. (B) Calibrator beads, coated with batch-defined increasing concentrations of mouse IgG1 antibody molecules (i, 4400; ii, 29 000; iii, 10 800), were stained with FITC-conjugated α-mouse antibody and analyzed by flow cytometry. (C) The geometric mean fluorescence intensity (MFI) of the 3 bead populations were plotted against the corresponding number of mouse IgG1 molecules. Linear regression revealed an R2 of 0.996. (D) Washed platelets were preincubated with AYP1 for 30 minutes at room temperature and incubated with FITC-conjugated α-mouse antibody. The MFI was determined by flow cytometry and used to quantify surface expression of CLEC-2 by extrapolation from the linear regression line of C. CLEC-2 copy number was determined for ten donors of diverse ethnic backgrounds (23-55 years of age) with a mean of 2016 ± 239 (mean ± standard deviation).
Quantification of platelet CLEC-2 expression. The number of surface copies of CLEC-2 per platelet was determined using the mouse α-human CLEC-2 antibody AYP1 and the Platelet Calibrator Kit from Biocytex. (A) Washed platelets were preincubated with 2.5 µg/mL AYP1 (saturating concentration) at room temperature for indicated times and subsequently incubated with a FITC-conjugated α-mouse antibody for another 15 minutes. Flow cytometric analysis shows that binding of AYP1 remains stable over the tested time period. (B) Calibrator beads, coated with batch-defined increasing concentrations of mouse IgG1 antibody molecules (i, 4400; ii, 29 000; iii, 10 800), were stained with FITC-conjugated α-mouse antibody and analyzed by flow cytometry. (C) The geometric mean fluorescence intensity (MFI) of the 3 bead populations were plotted against the corresponding number of mouse IgG1 molecules. Linear regression revealed an R2 of 0.996. (D) Washed platelets were preincubated with AYP1 for 30 minutes at room temperature and incubated with FITC-conjugated α-mouse antibody. The MFI was determined by flow cytometry and used to quantify surface expression of CLEC-2 by extrapolation from the linear regression line of C. CLEC-2 copy number was determined for ten donors of diverse ethnic backgrounds (23-55 years of age) with a mean of 2016 ± 239 (mean ± standard deviation).
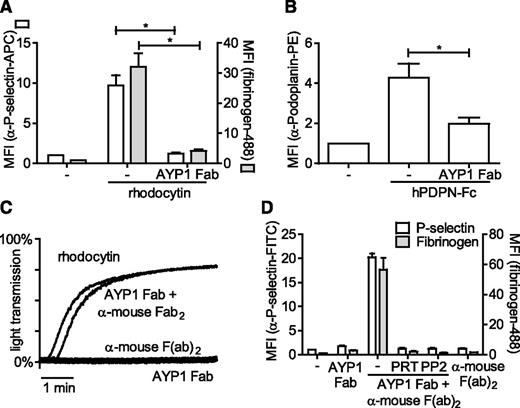
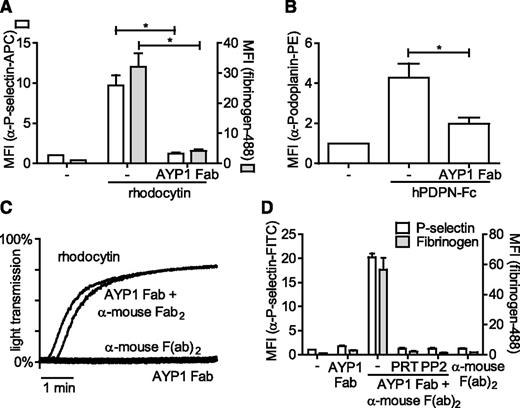
The full characterization of antibodies recognizing human platelets is complicated by expression of FcγRIIa, which can bind the Fc portion of antibodies. To address this, we generated Fab fragments of AYP1 and demonstrated that these antibody fragments also bound to human platelets. Furthermore, AYP1 Fab fragments were able to block platelet activation triggered by rhodocytin and podoplanin, suggesting that these ligands have binding sites on CLEC-2 that overlap with the AYP1 epitope (Figure 3A-B). Cross-linking of the AYP1 Fab fragments with a secondary antibody induced powerful platelet aggregation (Figure 3C) and enhanced fibrinogen binding and P-selectin expression (Figure 3D), which was blocked by inhibition of Src and Syk tyrosine kinases using PP2 and PRT-060318, respectively. This is consistent with CLEC-2-mediated platelet activation requiring receptor clustering and activation of Src and Syk tyrosine kinases.
Effect of AYP1 on CLEC-2 signaling. (A-B) Washed platelets were preincubated with 2.5 µg/mL AYP1 Fab fragments for 15 minutes at room temperature and stimulated with (A) 100 nM rhodocytin or (B) 10 µg/mL hPDPN-Fc for 15 minutes at 37°C. (A) Platelet activation by rhodocytin was determined by flow cytometric analysis of P-selectin expression and fibrinogen binding and (B) podoplanin binding using a phycoerythrin-conjugated α-human podoplanin antibody. (C) Aggregation of washed platelets induced by 100 nM rhodocytin or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments. Single incubation with either AYP1 Fab or α-mouse F(ab)2 fragments did not trigger aggregation. The traces are representative of 3 independent experiments. (D) Flow cytometric analysis of P-selectin expression and fibrinogen binding induced by cross-linking AYP1 Fab. Washed platelets were incubated with AYP1 Fab, α-mouse F(ab)2, or both for 15 minutes at 37°C and immediately analyzed. Preincubation with either the Syk inhibitor PRT-060318 (PRT; 5 µM) or the Src family kinase inhibitor PP2 (20 µM) for 10 minutes at room temperature prevented platelet activation induced by cross-linking AYP1 Fab. Data are presented as the ratio of MFI of treated over control platelets (n = 4).
Effect of AYP1 on CLEC-2 signaling. (A-B) Washed platelets were preincubated with 2.5 µg/mL AYP1 Fab fragments for 15 minutes at room temperature and stimulated with (A) 100 nM rhodocytin or (B) 10 µg/mL hPDPN-Fc for 15 minutes at 37°C. (A) Platelet activation by rhodocytin was determined by flow cytometric analysis of P-selectin expression and fibrinogen binding and (B) podoplanin binding using a phycoerythrin-conjugated α-human podoplanin antibody. (C) Aggregation of washed platelets induced by 100 nM rhodocytin or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments. Single incubation with either AYP1 Fab or α-mouse F(ab)2 fragments did not trigger aggregation. The traces are representative of 3 independent experiments. (D) Flow cytometric analysis of P-selectin expression and fibrinogen binding induced by cross-linking AYP1 Fab. Washed platelets were incubated with AYP1 Fab, α-mouse F(ab)2, or both for 15 minutes at 37°C and immediately analyzed. Preincubation with either the Syk inhibitor PRT-060318 (PRT; 5 µM) or the Src family kinase inhibitor PP2 (20 µM) for 10 minutes at room temperature prevented platelet activation induced by cross-linking AYP1 Fab. Data are presented as the ratio of MFI of treated over control platelets (n = 4).
AYP1 thus recognizes CLEC-2 in its native conformation and can be used to inhibit podoplanin and rhodocytin-mediated platelet activation. A second mouse mAb, AYP2, can be used to recognize the denatured form of CLEC-2 following SDS-PAGE.
CLEC-2 is not regulated by shedding or internalization upon platelet activation
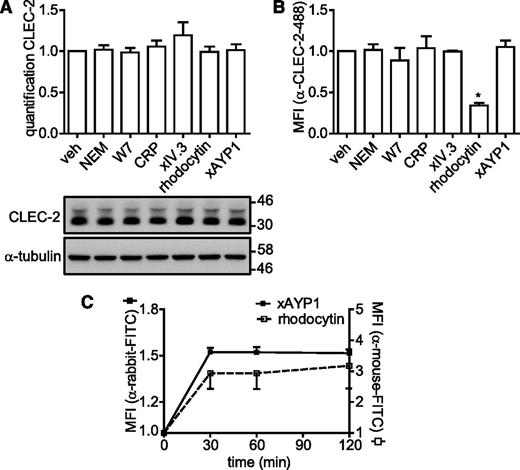
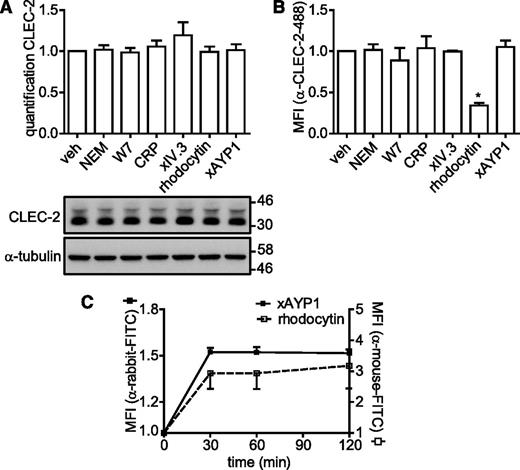
The newly generated antibodies were used to investigate whether CLEC-2 is regulated by proteolytic cleavage as is the case for GPVI and FcγRIIa. Washed platelets were incubated under a variety of conditions that cause down-regulation of GPVI and FcγRIIa, and in addition, were subjected to CLEC-2 stimulation by rhodocytin or cross-linked AYP1. Incubation with N-ethylmaleimide (NEM), a reagent that reacts with free sulfhydryl groups, or the calmodulin antagonist W7, which both induce metalloproteinase-mediated shedding of GPVI,26,36 had no effect on surface expression of CLEC-2 as measured by flow cytometry or cleavage of CLEC-2 as measured by western blot (Figure 4A-B). Binding of GPVI by collagen-related peptide (CRP), FcγRIIa by cross-linked IV.3 Fab fragments, or CLEC-2 by cross-linked AYP1 Fab also failed to alter surface expression or induce cleavage of CLEC-2. A reduced level of CLEC-2 expression was observed by flow cytometry but not by western blotting when platelets were stimulated by rhodocytin, which is likely to reflect competition between rhodocytin and AYP1 for binding to the C-type lectin-like receptor. To investigate this further, we monitored the time course of rhodocytin binding to platelets by flow cytometry using a previously described polyclonal Ab.30 Figure 4C shows that binding of rhodocytin is stable for up to 120 minutes. Similarly, cross-linked AYP1 Fab remained stably bound to CLEC-2 over the tested incubation time. In line with these findings, incubation with NEM, W7, or a rat α-mouse CLEC-2 mAb also failed to induce proteolytic cleavage of murine CLEC-2 (supplemental Figure 3). Together, these findings indicate that CLEC-2 is not shed or internalized from the platelet surface on autoactivation or in response to activation of GPVI and FcγRIIa, or agents that induce cleavage of the 2 ITAM receptors.
CLEC-2 is not shed or internalized following activation. (A-B) Washed platelets were incubated with vehicle control (veh; dimethylsulfoxide), NEM (2 mM), W7 (150 µM), CRP (10 µg/mL), IV.3 Fab (5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xIV.3), rhodocytin (100 nM), or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 1 hour at 37°C. (A) Platelet lysates were blotted and probed with AYP2. Membranes were subsequently stripped and reprobed with an antibody against α-tubulin. Quantification is presented as the mean ratio of treated over control platelets (n = 3). (B) Flow cytometric analysis of the surface expression of CLEC-2. After treatment, platelets were incubated with Alexa Fluor 488-conjugated AYP1 for 15 minutes at 37°C and immediately analyzed. Alexa Fluor 488-conjugated AYP1 Fab was used to assess CLEC-2 expression after AYP1 Fab cross-linking. (C) Analysis of surface-bound rhodocytin and cross-linked AYP1 Fab. Washed platelets were incubated with rhodocytin or xAYP1 for indicated times at 37°C. Surface-bound rhodocytin was determined by incubation with rabbit α-rhodocytin antibody, followed by incubation with F(ab)2 fragments of FITC-conjugated swine α-rabbit IgG. Surface-bound xAYP1 was assessed by incubation with F(ab)2 fragments of polyclonal FITC-conjugated sheep α-mouse IgG. Data are presented as the ratio of MFI of treated over control platelets (n = 5).
CLEC-2 is not shed or internalized following activation. (A-B) Washed platelets were incubated with vehicle control (veh; dimethylsulfoxide), NEM (2 mM), W7 (150 µM), CRP (10 µg/mL), IV.3 Fab (5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xIV.3), rhodocytin (100 nM), or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 1 hour at 37°C. (A) Platelet lysates were blotted and probed with AYP2. Membranes were subsequently stripped and reprobed with an antibody against α-tubulin. Quantification is presented as the mean ratio of treated over control platelets (n = 3). (B) Flow cytometric analysis of the surface expression of CLEC-2. After treatment, platelets were incubated with Alexa Fluor 488-conjugated AYP1 for 15 minutes at 37°C and immediately analyzed. Alexa Fluor 488-conjugated AYP1 Fab was used to assess CLEC-2 expression after AYP1 Fab cross-linking. (C) Analysis of surface-bound rhodocytin and cross-linked AYP1 Fab. Washed platelets were incubated with rhodocytin or xAYP1 for indicated times at 37°C. Surface-bound rhodocytin was determined by incubation with rabbit α-rhodocytin antibody, followed by incubation with F(ab)2 fragments of FITC-conjugated swine α-rabbit IgG. Surface-bound xAYP1 was assessed by incubation with F(ab)2 fragments of polyclonal FITC-conjugated sheep α-mouse IgG. Data are presented as the ratio of MFI of treated over control platelets (n = 5).
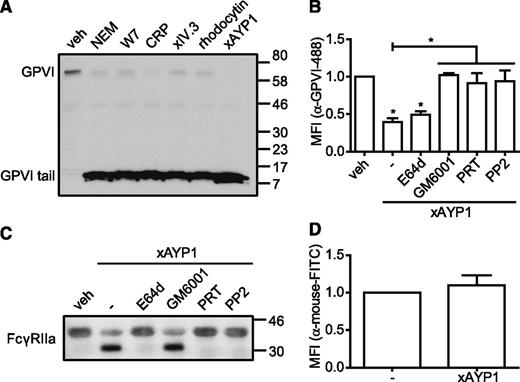
Activation of CLEC-2 induces proteolytic cleavage of GPVI and FcγRIIa
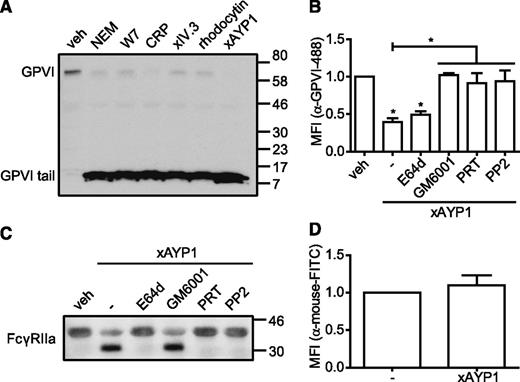
GPVI and FcγRIIa are regulated by metalloproteinase-mediated ectodomain shedding24 and by intracellular calpain-mediated cleavage,25,37 respectively. We therefore investigated whether CLEC-2 ligation also affects expression of GPVI by probing platelet lysates with an α-human GPVI cytoplasmic tail antibody that detects full-length (∼62 kDa) and the proteolytically cleaved remnant (∼10 kDa) of platelet-associated GPVI and of FcγRIIa by probing with a western blotting antibody against the low affinity immune receptor (Figure 5). In agreement with previous reports,25,26 incubation with NEM, W7, CRP, or cross-linked IV.3 Fab induced shedding of GPVI (Figure 5A). Similarly, we show that CLEC-2 activation by rhodocytin or by AYP1 Fab cross-linking induces proteolysis of GPVI and concomitant generation of the remnant GPVI fragment. CLEC-2–mediated shedding of GPVI was confirmed by flow cytometry (Figure 5B). Further, proteolysis of GPVI in response to CLEC-2 was inhibited by the broad-range metalloproteinase inhibitor, GM6001, and by selective inhibition of Src (PP2) and Syk (PRT-060318) kinases (Figure 5B). Preincubation with the membrane-permeable calpain inhibitor E64d did not prevent CLEC-2–induced shedding of GPVI (Figure 5B). Western blot analysis revealed that activation of CLEC-2 also resulted in proteolytic cleavage of FcγRIIa, which was inhibited by E64d, PRT-060318, and PP2, but not by GM6001 (Figure 5C). No loss of FcγRIIa was detected by flow cytometry (Figure 5D), confirming earlier findings that the extracellular domain remains platelet associated after proteolysis.25 These results collectively demonstrate that CLEC-2 signaling induces metalloproteinase-dependent shedding of GPVI and calpain-dependent proteolytic cleavage of FcγRIIa and that this proteolysis requires activation of Src and Syk tyrosine kinases.
Activation of CLEC-2 leads to proteolytic cleavage of GPVI and FcγRIIa. (A) Washed platelets were incubated with vehicle control (veh; dimethylsulfoxide), NEM (2 mM), W7 (150 µM), CRP (10 µg/mL), IV.3 Fab (5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xIV.3), rhodocytin (100 nM), or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 1 hour at 37°C. Platelet lysates were blotted and probed with an antibody against the cytoplasmic tail of GPVI, which detects both full-length and the cytoplasmic tail remnant of GPVI. The blot is representative for ≥3 independent experiments. (B) Flow cytometric analysis of the surface expression of GPVI. Washed platelets were preincubated with the calpain inhibitor E64d (100 µM), the metalloproteinase inhibitor GM6001 (100 µM), the Syk inhibitor PRT-060318 (PRT; 5 µM), or the Src family kinase inhibitor PP2 (20 µM) for 10 minutes at room temperature, followed by incubation with AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 15 minutes at 37°C. After treatment, platelets were incubated with Alexa Fluor 488-conjugated α-GPVI antibody 1G5 for 15 minutes at 37°C and immediately analyzed. (C) Washed platelets incubated under the conditions of B were blotted and probed with mouse α-human FcγRIIa-biotin. The blot is representative for ≥3 independent experiments. (D) Flow cytometric analysis of FcγRIIa surface expression on platelets after AYP1 Fab cross-linking. Data are presented as the ratio of MFI of treated over control platelets (n = 3).
Activation of CLEC-2 leads to proteolytic cleavage of GPVI and FcγRIIa. (A) Washed platelets were incubated with vehicle control (veh; dimethylsulfoxide), NEM (2 mM), W7 (150 µM), CRP (10 µg/mL), IV.3 Fab (5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xIV.3), rhodocytin (100 nM), or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 1 hour at 37°C. Platelet lysates were blotted and probed with an antibody against the cytoplasmic tail of GPVI, which detects both full-length and the cytoplasmic tail remnant of GPVI. The blot is representative for ≥3 independent experiments. (B) Flow cytometric analysis of the surface expression of GPVI. Washed platelets were preincubated with the calpain inhibitor E64d (100 µM), the metalloproteinase inhibitor GM6001 (100 µM), the Syk inhibitor PRT-060318 (PRT; 5 µM), or the Src family kinase inhibitor PP2 (20 µM) for 10 minutes at room temperature, followed by incubation with AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) for 15 minutes at 37°C. After treatment, platelets were incubated with Alexa Fluor 488-conjugated α-GPVI antibody 1G5 for 15 minutes at 37°C and immediately analyzed. (C) Washed platelets incubated under the conditions of B were blotted and probed with mouse α-human FcγRIIa-biotin. The blot is representative for ≥3 independent experiments. (D) Flow cytometric analysis of FcγRIIa surface expression on platelets after AYP1 Fab cross-linking. Data are presented as the ratio of MFI of treated over control platelets (n = 3).
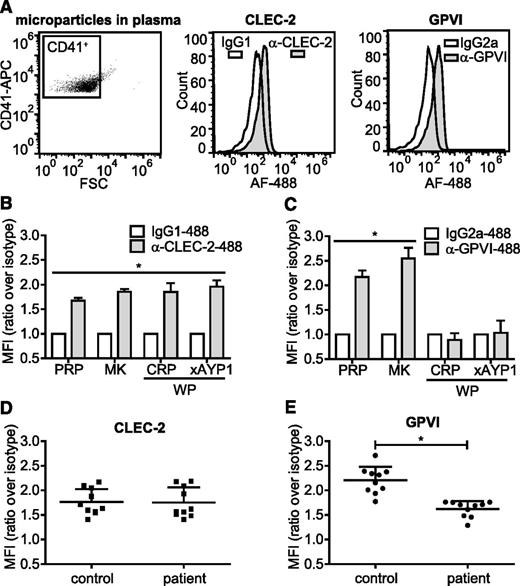
Microparticles from activated platelets retain CLEC-2 but lose GPVI expression
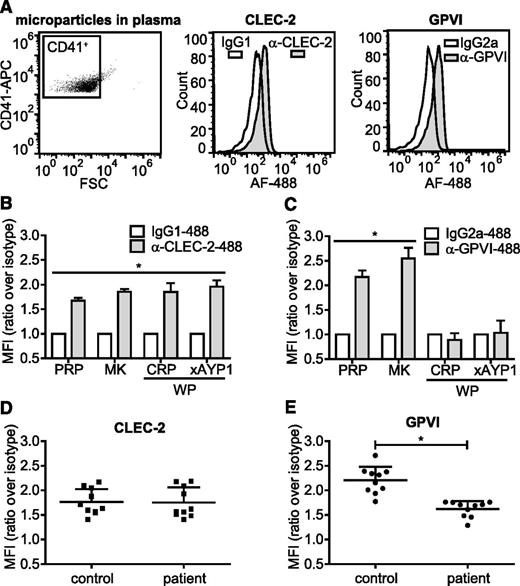
Megakaryocytes and activated platelets shed small submicron fragments into the circulation, accounting for between 70% and 90% of microparticles in the blood stream38-40 as shown by positive staining for the megakaryocyte/platelet integrin subunit αIIb (CD41). In healthy individuals, the majority of circulating CD41+ microparticles originates from megakaryocytes rather than from activated platelets.41,42 We analyzed CD41+ microparticles isolated from plasma, primary human megakaryocytes, and activated platelets for surface expression of CLEC-2 and GPVI. CD41+ microparticles isolated from plasma of healthy volunteers expressed CLEC-2 and GPVI (Figure 6A). These microparticles stained negative for CD45, indicating that they were not fused with microparticles of leukocyte origin (supplemental Figure 5A). A similar level of CLEC-2 and GPVI expression was found on CD41+ microparticles isolated from megakaryocytes cultured for 12 days (Figure 6B-C). In contrast, microparticles derived from platelets activated with either CRP or AYP1 Fab cross-linking contained CLEC-2 but not GPVI, suggesting that treatment of microparticle formation in platelets results in proteolytic cleavage of the collagen receptor. Incubation of CD41+ microparticles isolated from plasma with CRP or cross-linked AYP1 Fab failed to induce loss of either receptor, indicating that microparticles are not capable of GPVI proteolysis (supplemental Figure 5B). Microparticles formed by incubating platelets with concentrations of CRP or AYP1 Fab that do not induce ful activation, or microparticles formed during storage of platelet concentrates, also retained CLEC-2 but lost GPVI expression (supplemental Figure 5C-E). Together, these findings indicate that the presence of GPVI can be used to distinguish microparticles derived from either megakaryocytes or activated platelets.
Microparticles from activated platelets maintain CLEC-2, but lose GPVI expression. (A) Microparticles were isolated from fresh PRP as described in the methods section and (left) CD41+ microparticles were analyzed for (center) CLEC-2 and (right) GPVI expression using Alexa Fluor 488-conjugated α-CLEC-2 antibody AYP1 and α-GPVI antibody 1G5, respectively. True positivity was determined using isotype-matched controls. (B-C) CD41+ microparticles isolated from PRP from a 12-day megakaryocyte (MK) culture or from washed platelets (WP) that were incubated for 1 hour at 37°C with CRP (10 µg/mL) or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) were analyzed for (B) CLEC-2 and (C) GPVI expression. Data are presented as the ratio of MFI of CLEC-2 or GPVI over isotype controls (n = 4). (D-E) Analysis of microparticles from healthy controls and patients with rheumatoid arthritis. CD41+ microparticles isolated from fresh PRP were analyzed for (D) CLEC-2 and (E) GPVI expression. Data are presented as the ratio of MFI over isotype controls (mean ± standard deviation; n = 10).
Microparticles from activated platelets maintain CLEC-2, but lose GPVI expression. (A) Microparticles were isolated from fresh PRP as described in the methods section and (left) CD41+ microparticles were analyzed for (center) CLEC-2 and (right) GPVI expression using Alexa Fluor 488-conjugated α-CLEC-2 antibody AYP1 and α-GPVI antibody 1G5, respectively. True positivity was determined using isotype-matched controls. (B-C) CD41+ microparticles isolated from PRP from a 12-day megakaryocyte (MK) culture or from washed platelets (WP) that were incubated for 1 hour at 37°C with CRP (10 µg/mL) or AYP1 Fab (2.5 µg/mL) cross-linked with 20 µg/mL α-mouse Fab-specific F(ab)2 fragments (xAYP1) were analyzed for (B) CLEC-2 and (C) GPVI expression. Data are presented as the ratio of MFI of CLEC-2 or GPVI over isotype controls (n = 4). (D-E) Analysis of microparticles from healthy controls and patients with rheumatoid arthritis. CD41+ microparticles isolated from fresh PRP were analyzed for (D) CLEC-2 and (E) GPVI expression. Data are presented as the ratio of MFI over isotype controls (mean ± standard deviation; n = 10).
Elevated platelet-derived microparticle levels are associated with a number of cardiovascular and inflammatory diseases, including arterial thrombosis,43,44 heparin-induced thrombocytopenia,45 immune thrombocytopenia,46 malaria infection,47 AIDS,48 and rheumatoid arthritis.22,49 To verify whether the presence of GPVI on microparticles allows discrimination of megakaryocyte- from platelet-derived microparticles, we isolated microparticles from peripheral blood plasma samples from patients with rheumatoid arthritis and analyzed CLEC-2 and GPVI levels on CD41+ microparticles. As expected, the total number of CD41+ microparticles was increased in the patient group (Table 1). Levels of CLEC-2 were similar to levels on microparticles from healthy controls, but GPVI levels were markedly decreased (Figure 6D-E; Table 1). This reduction agrees with an increase in levels of plasma soluble GPVI (Table 1). These results suggest that measurement of GPVI levels on CD41+/CLEC-2+ microparticles in plasma can be used to determine whether patients have increased levels of microparticles derived from activated platelets.
Discussion
In the present study, we generated human mAbs to CLEC-2 and demonstrated the following: (1) in peripheral blood of healthy individuals, CLEC-2 is restricted to platelets with a copy number of 2016 ± 76 per cell; (2) CLEC-2 is not regulated by proteolytic cleavage or internalization following autoactivation or in response to activation of the ITAM receptors GPVI and FcγRIIa; (3) activation of CLEC-2 leads to Src and Syk kinase-mediated proteolytic cleavage of GPVI and FcγRIIa; (4) megakaryocyte-derived microparticles express CLEC-2 and GPVI, whereas microparticles derived from activated platelets lose GPVI; and (5) the greater expression of CLEC-2 relative to GPVI on microparticles and increased soluble GPVI levels in patients with rheumatoid arthritis provides evidence of activation of platelets in the circulation during this inflammatory disorder.
CLEC-2 was first identified during a bioinformatic screen of human myeloid cells for C-type lectin-like receptors, with mRNA transcripts reported in monocytes, granulocytes, and dendritic cells.50 A later study of the human transcriptome found high levels of CLEC-2 mRNA in bone marrow, liver, and whole blood, but not in leukocytes (supplemental Figure 2).51 In agreement with this study, we observed expression of CLEC-2 on platelets but not on myeloid and lymphocyte populations isolated from peripheral blood. Del Rey and colleagues were also unable to detect CLEC-2 expression on cells other than platelets in synovial fluid of rheumatoid arthritis patients and failed to detect the receptor on human immature and mature dendritic cells.52 Expression of CLEC-2 on human cells other than platelets has thus far only been identified on the monocytic leukemia THP-1 cell line.53 On the other hand, studies in mice using a selective mAb, 17D9, have reported expression of CLEC-2 on a broad range of hematopoietic cells, including neutrophils, natural killer cells, tissue-resident dendritic cells, and macrophages, along with enhanced expression during inflammatory insults.54-56 The functional significance of this differential distribution is not known.
Of considerable interest in this study is the observation that, in contrast to the related ITAM receptors GPVI and FcγRIIa, CLEC-2 is not regulated by proteolytic cleavage on activation or in response to activation of either of the ITAM receptors. Shedding of GPVI is mediated by a disintegrin and metalloproteinase family pathway.57 In contrast, proteolysis of FcγRIIa occurs via activation of calpain. Both receptors are also cleaved after treatment with the calmodulin inhibitor W7 to dissociate calmodulin from a binding site within the cytoplasmic tails of GPVI and FcγRIIa.25 There is no identifiable calmodulin binding motif within the cytoplasmic tail of CLEC-2.
These observations give rise to the question as to why the levels of GPVI and FcγRIIa are controlled by proteolytic cleavage but not those of CLEC-2. Down-regulation of GPVI and FcγRIIa by proteolysis may help to limit uncontrolled platelet activation (and thereby thrombus formation) in the circulation either in response to weak constitutive signaling by either receptor58,59 or, in the case of FcγRIIa, in response to contact with immune complexes. It could also be important to limit the time and extent of platelet activation, for example, in relation to the role of GPVI in the maintenance of vascular integrity, although this would also apply to CLEC-2.21 Chronic ligand engagement of GPVI could contribute to persistent platelet activation and inflammation in the vessel wall, as observed in rheumatoid arthritis, deep vein thrombosis, and atherosclerosis, and this would be countered by shedding.
One potential explanation as to why CLEC-2 is not subject to the same regulatory pathways could be related to the absence of podoplanin in the vasculature or in the immediate vicinity of damage to the vessel wall. Furthermore, functional engagement of CLEC-2 and podoplanin may require a sustained interaction. In this context, it may be important to recognize CLEC-2 as both a signaling receptor and a ligand that regulates podoplanin signaling. The role of CLEC-2 and podoplanin in prevention of blood-lymphatic mixing18-20,60 and in maintenance of integrity in high endothelial venules61 may require sustained activation of podoplanin to permit changes such as gene regulation to occur. At the same time, it is possible that the shedding of GPVI by CLEC-2 may represent an important mechanism to limit thrombus growth during contact between the 2 vasculatures. Although the present results demonstrate that CLEC-2 is not down-regulated on human platelets in vitro, it is noteworthy that down-regulation of CLEC-2 has been described in mouse platelets in vivo on exposure to a rat α-mouse CLEC-2 mAb, INU1.14 The molecular basis of this down-regulation and its physiological significance are unclear. We showed that the binding of CLEC-2 induces proteolysis of GPVI and FcγRIIa. In the same context, GPVI is capable of inducing proteolysis of FcγRIIa and vice versa.25 The physiological implication of this is unclear, as this could reflect the shared signaling mechanisms by all 3 receptors. Shedding of GPVI by CLEC-2 during lympatic development may represent an important mechanism to limit thrombus growth during this important physiological process. Alternatively, CLEC-2–induced proteolysis of GPVI and FcγRIIa could regulate a novel pathway of regulation of platelet function.
Platelet-derived microparticles are generated in response to a range of pathologic processes, and their determination and characterization may provide insights into the molecular mechanism of disease.22,43-49 They may also play physiological roles in processes ascribed to platelet function. For example, microparticles could potentially mediate some of the functions ascribed to CLEC-2 on platelets in processes such as development of the lymphatics and lymph nodes.62 Using flow cytometry, we demonstrate that megakaryocyte-derived microparticles in plasma of healthy individuals express CLEC-2 and GPVI, whereas GPVI is absent from microparticles derived from activated platelets. It has recently been shown that megakaryocyte-derived microparticles can be distinguished from platelet-derived microparticles by the absence of surface-expressed P-selectin and lysosome-associated membrane protein-1 and by the presence of full-length filamin A.41 In line with our study, microvesicles derived from mouse megakaryocytes also stained positive for GPVI.41 As P-selectin and lysosome-associated membrane protein-1 are also expressed by other cells63,64 and determination of filamin A requires isolation of microparticles and western blotting, screening for CD41+/CLEC-2+/GPVI− microparticles by flow cytometry may provide an attractive alternative to quantitate platelet-derived microparticles in circulation. This is illustrated in the present study by analysis of plasma samples from patients with the chronic inflammatory disease rheumatoid arthritis, which is associated with increased microparticle production.22,49 Increased microparticle production in rheumatoid arthritis patients has been attributed to collagen-mediated platelet activation around the vasculature of the joint, which is in close contact with fibroblast-like synoviocytes and extracellular matrix.22 Importantly, in the present study, we show that the majority of the circulating microparticles lack GPVI but retain CLEC-2 and CD41 expression in this inflammatory disorder, suggesting that they are partly derived from megakaryocytes and part from platelet activation. The reduced levels of GPVI expression on microparticles and elevated levels of soluble GPVI in patients with rheumatoid arthritis support the role of these measurements as biomarkers of disease.
In summary, we describe a novel mouse mAb against human CLEC-2, AYP1, and use this to demonstrate the restricted distribution of CLEC-2 to platelets, the resistance of the C-type lectin receptor to proteolysis, and the CLEC-2–induced cleavage of GPVI and FcγRIIa. The observation that microparticles derived from activated platelets retain CLEC-2 but lose GPVI highlights the potential use of measurement of surface expression of platelet receptors to screen for platelet activation in a wide variety of cardiovascular and inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Clara Yates, Guillaume Desanti, Danai Bem, and Stephanie Watson for useful discussions and technical assistance. The authors also thank Chris O’Callaghan for providing the vector used for recombinant CLEC-2 production.
This work was supported by the British Heart Foundation (RG/09/007/27917; PG/10/36/02) and Wellcome Trust (093994). E.E.G. and R.K.A. receive support from the National Health and Medical Research Council of Australia. E.E.G. is also supported by the Institute of Advanced Studies from the University of Birmingham. P.H. is supported by the Healing Foundation. S.P.W. holds a British Heart Foundation chair (CH/03/003).
This article presents independent research funded by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Centre (partnership between University Hospitals Birmingham National Health Service Foundation Trust, the University of Birmingham, and the Royal Centre for Defence Medicine). The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Authorship
Contribution: E.G. designed and performed experiments, analyzed and interpreted results, made the figures, and wrote the manuscript; A.Y.P., J.J.G.-F., O.A., S.M., and J.M. performed experiments, analyzed and interpreted results, and commented on the manuscript; M.R.D. assisted in the generation of the CLEC-2 mAbs and commented on the manuscript; E.E.G., R.K.A., G.B.N., and C.D.B. interpreted results, provided essential reagents, and commented on the manuscript; P.H. designed experiments, interpreted results, and commented on the manuscript; and S.P.W. designed the research, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steve P. Watson, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; e-mail: s.p.watson@bham.ac.uk.